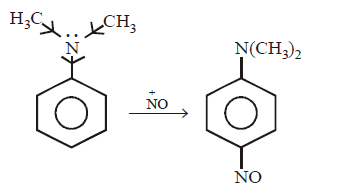
11. Which of the following can undergo electrophilic substitution when treated with nitrous acid at $${0^ \circ }C?$$
A
$${C_6}{H_5}N{H_2}$$
B
$${C_6}{H_5}NHC{H_3}$$
C
$${C_6}{H_5}N{\left( {C{H_3}} \right)_2}$$
D
$${\text{None}}$$
Answer :
$${C_6}{H_5}N{\left( {C{H_3}} \right)_2}$$
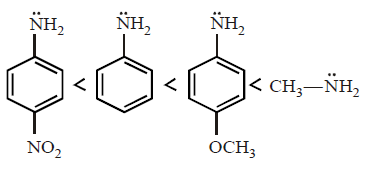
12. Arrange the following amines in the order of increasing basicity.
A


B


C


D


Answer :


13. The correct order of increasing basic nature for the bases $$N{H_3},C{H_3}N{H_2}\,{\text{and}}\,{\left( {C{H_3}} \right)_2}NH$$ is
A
$$\left( {C{H_3}} \right)NH < N{H_3} < C{H_3}N{H_2}$$
B
$$N{H_3} < C{H_3}N{H_2} < {\left( {C{H_3}} \right)_2}NH$$
C
$$C{H_3}N{H_2} < {\left( {C{H_3}} \right)_2}NH < N{H_3}$$
D
$$C{H_3}N{H_2} < N{H_3} < {\left( {C{H_3}} \right)_2}NH$$
Answer :
$$N{H_3} < C{H_3}N{H_2} < {\left( {C{H_3}} \right)_2}NH$$
14. Primary nitro compounds react with nitrous acid to form nitrolic acids which dissolve in $$NaOH$$ giving
A
yellow solution
B
blue solution
C
colourless solution
D
red solution
Answer :
red solution
15. Which one of the following methods is neither meant for the synthesis nor for separation of amines?
A
Curtius reaction
B
Wurtz reaction
C
Hofmann method
D
Hinsberg method
Answer :
Wurtz reaction
16. When aniline reacts with oil of bitter almonds $$\left( {{C_6}{H_5}CHO} \right)$$ condensation takes place and benzal derivative is formed. This is known as
A
Million's base
B
Schiff's reagent
C
Schiff's base
D
Benedict's reagent
Answer :
Schiff's base
17.
Consider the following sequence of reactions
\[\text{Compound}\left[ A \right]\xrightarrow{\text{Reduction}}\left[ B \right]\] \[\xrightarrow{HN{{O}_{2}}}C{{H}_{3}}C{{H}_{2}}OH\]
The compound $$[A]$$ is
A
$$C{H_3}C{H_2}CN$$
B
$$C{H_3}N{O_2}$$
C
$$C{H_3}NC$$
D
$$C{H_3}CN$$
Answer :
$$C{H_3}CN$$
18. 3, 5 - dibromotoluene can be best synthesised by
A
 \[\xrightarrow{B{{r}_{2}}/{{H}_{2}}O}\,\,\,\xrightarrow[{{H}_{3}}P{{O}_{2}}]{HN{{O}_{2}}}\]
\[\xrightarrow{B{{r}_{2}}/{{H}_{2}}O}\,\,\,\xrightarrow[{{H}_{3}}P{{O}_{2}}]{HN{{O}_{2}}}\]
 \[\xrightarrow{B{{r}_{2}}/{{H}_{2}}O}\,\,\,\xrightarrow[{{H}_{3}}P{{O}_{2}}]{HN{{O}_{2}}}\]
\[\xrightarrow{B{{r}_{2}}/{{H}_{2}}O}\,\,\,\xrightarrow[{{H}_{3}}P{{O}_{2}}]{HN{{O}_{2}}}\] B
 \[\xrightarrow[{{H}_{2}}S{{O}_{4}}]{\text{Fuming}\,\,HN{{O}_{3}}}\,\,\,\xrightarrow{N{{H}_{3}}}\,\,\,\xrightarrow[CuBr]{HONO}\]
\[\xrightarrow[{{H}_{2}}S{{O}_{4}}]{\text{Fuming}\,\,HN{{O}_{3}}}\,\,\,\xrightarrow{N{{H}_{3}}}\,\,\,\xrightarrow[CuBr]{HONO}\]
 \[\xrightarrow[{{H}_{2}}S{{O}_{4}}]{\text{Fuming}\,\,HN{{O}_{3}}}\,\,\,\xrightarrow{N{{H}_{3}}}\,\,\,\xrightarrow[CuBr]{HONO}\]
\[\xrightarrow[{{H}_{2}}S{{O}_{4}}]{\text{Fuming}\,\,HN{{O}_{3}}}\,\,\,\xrightarrow{N{{H}_{3}}}\,\,\,\xrightarrow[CuBr]{HONO}\] C
 \[\xrightarrow[\Delta ]{B{{r}_{2}}/Fe}\]
\[\xrightarrow[\Delta ]{B{{r}_{2}}/Fe}\]
 \[\xrightarrow[\Delta ]{B{{r}_{2}}/Fe}\]
\[\xrightarrow[\Delta ]{B{{r}_{2}}/Fe}\] D
 \[\xrightarrow[\Delta ]{C{{H}_{3}}Cl/AlC{{l}_{3}}}\]
\[\xrightarrow[\Delta ]{C{{H}_{3}}Cl/AlC{{l}_{3}}}\]
 \[\xrightarrow[\Delta ]{C{{H}_{3}}Cl/AlC{{l}_{3}}}\]
\[\xrightarrow[\Delta ]{C{{H}_{3}}Cl/AlC{{l}_{3}}}\]
Answer :
 \[\xrightarrow{B{{r}_{2}}/{{H}_{2}}O}\,\,\,\xrightarrow[{{H}_{3}}P{{O}_{2}}]{HN{{O}_{2}}}\]
\[\xrightarrow{B{{r}_{2}}/{{H}_{2}}O}\,\,\,\xrightarrow[{{H}_{3}}P{{O}_{2}}]{HN{{O}_{2}}}\]
 \[\xrightarrow{B{{r}_{2}}/{{H}_{2}}O}\,\,\,\xrightarrow[{{H}_{3}}P{{O}_{2}}]{HN{{O}_{2}}}\]
\[\xrightarrow{B{{r}_{2}}/{{H}_{2}}O}\,\,\,\xrightarrow[{{H}_{3}}P{{O}_{2}}]{HN{{O}_{2}}}\] 19. Which of the following orders is true regarding the basic nature of $$ - N{H_2}$$ group?
A
$$o$$ - Toluidine > Aniline > $$o$$ - Nitroaniline
B
$$o$$ - Toluidine < Aniline > $$o$$ - Nitroaniline
C
$$o$$ - Toluidine < Aniline < $$o$$ - Nitroaniline
D
$$o$$ - Toluidine > Aniline < $$o$$ - Nitroaniline
Answer :
$$o$$ - Toluidine < Aniline > $$o$$ - Nitroaniline
20. Best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is
A
Hofmann bromamide reaction
B
Gabriel phthalimide synthesis
C
Sandmeyer reaction
D
reaction with $$N{H_3}$$
Answer :
Gabriel phthalimide synthesis