111. The gas leaked from a storage tank of the Union Carbide plant in Bhopal gas tragedy was :
A
Methyl isocyanate
B
Methylamine
C
Ammonia
D
Phosgene
Answer :
Methyl isocyanate
112.
Choose the correct option by arranging the following compounds in increasing order of basicity :
$$C{H_3}N{H_2},{\left( {C{H_3}} \right)_2}NH,N{H_3},$$ $${C_6}{H_5}N{H_2}$$
A
$${C_6}{H_5}N{H_2} < N{H_3} < {\left( {C{H_3}} \right)_2}NH$$ $$ < C{H_3}N{H_2}$$
B
$$C{H_3}N{H_2} < {\left( {C{H_3}} \right)_2}NH < N{H_3}$$ $$ < {C_6}{H_5}N{H_2}$$
C
$${C_6}{H_5}N{H_2} < N{H_3} < C{H_3}N{H_2}$$ $$ < {\left( {C{H_3}} \right)_2}NH$$
D
$${\left( {C{H_3}} \right)_2}NH < C{H_3}N{H_2} < N{H_3}$$ $$ < {C_6}{H_5}N{H_2}$$
Answer :
$${C_6}{H_5}N{H_2} < N{H_3} < C{H_3}N{H_2}$$ $$ < {\left( {C{H_3}} \right)_2}NH$$
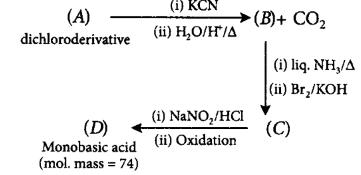
114. A dichloroderivative $$(A)$$ on treating with $$KCN$$ followed by acid hydrolysis and heating gives a monobasic acid $$(B)$$ along with liberation of $$C{O_2}.$$ $$(B)$$ on heating with liquid ammonia followed by treating with $$B{r_2}/KOH$$ gives $$(C)$$ which on treating with $$NaN{O_2}$$ and $$HCl$$ at low temperature followed by oxidation gives a monobasic acid $$(D)$$ having molecular mass 74. $$'C'$$ in the whole process would be
A
ethyl amine
B
propyl amine
C
$$tert$$ - butyl amine
D
cyclopentyl amine
Answer :
propyl amine
115. Which of the following reactions is not correctly matched?
A
Reaction used to convert amide into primary amine with one carbon atom less - Hoffmann bromamide reaction
B
Reaction used to convert primary amines into isocyanides - Carbylamine reaction
C
Reaction used to distinguish primary, secondary and tertiary amines - Hinsberg's reaction
D
Preparation of primary amines using phthalimide - Victor Meyer's synthesis
Answer :
Preparation of primary amines using phthalimide - Victor Meyer's synthesis
116.
The correct increasing order of basic strength for the following compounds is _________.

A
II < III < I
B
III < I < II
C
IIII < II < I
D
II < I < III
Answer :
II < I < III
117. Hydrolysis of $$C{H_3}C{H_2}N{O_2}$$ with $$85\% \,{H_2}S{O_4}$$ gives
A
$$C{H_3}C{H_2}OH$$
B
$${C_2}{H_6}$$
C
$$C{H_3}CH = NOH$$
D
$$C{H_3}COOH$$
Answer :
$$C{H_3}COOH$$
118. Aniline is reacted with bromine water and the resulting product is treated with an aqueous solution of sodium nitrite in presence of dilute $$HCl.$$ The compound so formed is converted into tetrafluoroborate which is subsequently heated dry. The final product is
A
$$p$$ - Bromofluorobenzene
B
$$p$$ - Bromoaniline
C
2, 4, 6 - Tribromofluorobenzene
D
1, 3, 5 - Tribromobenzene
Answer :
2, 4, 6 - Tribromofluorobenzene
119. Which of the following tests is suitable to differentiate between aniline and benzylamine?
A
Aniline gives dye test on diazotisation and reaction with $$\beta $$ - naphthol while benzylamine gives alcohol.
B
Benzylamine gives green dye with $$\beta $$ - naphthol after diazotisation while aniline gives orange dye.
C
Aniline gives carbylamine reaction while benzylamine does not.
D
Benzylamine gives carbylamine reaction while aniline does not.
Answer :
Aniline gives dye test on diazotisation and reaction with $$\beta $$ - naphthol while benzylamine gives alcohol.
120.
Aniline in a set of reactions yielded a product
 \[\xrightarrow[HCl]{NaN{{O}_{2}}}A\xrightarrow{CuCN}B\xrightarrow[Ni]{{{H}_{2}}}\] \[C\xrightarrow{HN{{O}_{2}}}D\]
\[\xrightarrow[HCl]{NaN{{O}_{2}}}A\xrightarrow{CuCN}B\xrightarrow[Ni]{{{H}_{2}}}\] \[C\xrightarrow{HN{{O}_{2}}}D\]
The structure of the product $$D$$ would be
A
$${C_6}{H_5}C{H_2}N{H_2}$$
B
$${C_6}{H_5}NHC{H_2}C{H_3}$$
C
$${C_6}{H_5}NHOH$$
D
$${C_6}{H_5}C{H_2}OH$$
Answer :
$${C_6}{H_5}C{H_2}OH$$