21.
In the following reaction,  \[\xrightarrow{\frac{O{{H}^{-}}}{\Delta }}X\]
\[\xrightarrow{\frac{O{{H}^{-}}}{\Delta }}X\]
The organic product $$X$$ has the structure
A


B


C


D


Answer :


22. Which of the following is amphoteric in nature?
A
$$C{H_3}N{H_2}$$
B
$$C{H_3}NHC{H_3}$$
C
$$C{H_3}CON{H_2}$$
D
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\mathop{-N-}}\,C{{H}_{3}}\]
Answer :
$$C{H_3}CON{H_2}$$
23. The most convenient method to prepare an amine containing one carbon atom less is
A
Gabriel phthalimide synthesis
B
reductive amination of aldehydes
C
Hofmann bromamide reaction
D
reduction of isonitriles
Answer :
Hofmann bromamide reaction
24. Among the following, the strongest base is
A
$${C_6}{H_5}N{H_2}$$
B
$$p{\text{ - }}N{O_2}.{C_6}{H_4}N{H_2}$$
C
$$m{\text{ - }}N{O_2}.{C_6}{H_4}.N{H_2}$$
D
$${C_6}{H_5}C{H_2}N{H_2}$$
Answer :
$${C_6}{H_5}C{H_2}N{H_2}$$
25.
 \[\xrightarrow{A{{c}_{2}}O}A\xrightarrow[C{{H}_{3}}COOH]{B{{r}_{2}}}B\xrightarrow[{{H}^{+}}]{{{H}_{2}}O}C\]
\[\xrightarrow{A{{c}_{2}}O}A\xrightarrow[C{{H}_{3}}COOH]{B{{r}_{2}}}B\xrightarrow[{{H}^{+}}]{{{H}_{2}}O}C\]
Product $$C$$ would be
A


B


C


D


Answer :


26.
The correct stability order of the following resonance
structures is
$$\eqalign{
& \mathop {{H_2}C = \mathop N\limits^ + = \mathop N\limits^ - }\limits_{\left( {\text{I}} \right)} \cr
& \mathop {{H_2}\mathop C\limits^ + - N = \mathop N\limits^ - }\limits_{\left( {{\text{II}}} \right)} \cr
& \mathop {{H_2}\mathop C\limits^ - - \mathop N\limits^ + \equiv N}\limits_{\left( {{\text{III}}} \right)} \cr
& \mathop {{H_2}\mathop C\limits^ - - N = \mathop N\limits^ + }\limits_{\left( {{\text{IV}}} \right)} \cr} $$
A
(I) > (II) > (IV) > (III)
B
(I) > (III) > (II) > (IV)
C
(II) > (I) > (III) > (IV)
D
(III) > (I) > (IV) > (II)
Answer :
(I) > (III) > (II) > (IV)
27.
Which of the following reagents will be useful to distinguish between  and
and 
A
Dilute $$HCl$$
B
\[{{C}_{6}}{{H}_{5}}S{{O}_{2}}\] and \[O{{H}^{-}}/{{H}_{2}}O\]
C
$$HONO$$ then \[\beta \] - naphthol
D
\[AgN{{O}_{3}}\] in \[{{H}_{2}}O\]
Answer :
$$HONO$$ then \[\beta \] - naphthol
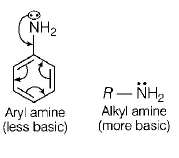
28. The correct statement regarding the basicity of arylamines is
A
Arylamines are generally more basic than alkylamines because the nitrogen lone-pair electrons are not delocalized by interaction with the aromatic ring $$\pi $$ - electron system
B
Arylamines are generally more basic than alkylamines because of aryl group
C
Arylamines are generally more basic than alkylamines, because the nitrogen atom in arylamines is $$sp$$ - hybridized
D
Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring $$\pi $$ - electron system
Answer :
Arylamines are generally less basic than alkylamines because the nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring $$\pi $$ - electron system
29.
The correct order of increasing basic nature of the following bases is

A
2 < 5 < 1 < 3 < 4
B
5 < 2 < 1 < 3 < 4
C
2 < 5 < 1 < 4 < 3
D
5 < 2 < 1 < 4 < 3
Answer :
2 < 5 < 1 < 3 < 4
30. Amongst the following, the strongest base in aqueous medium is _________.
A
$$C{H_3}N{H_2}$$
B
$$NCC{H_2}N{H_2}$$
C
$${\left( {C{H_3}} \right)_2}NH$$
D
$${C_6}{H_5}NHC{H_3}$$
Answer :
$${\left( {C{H_3}} \right)_2}NH$$