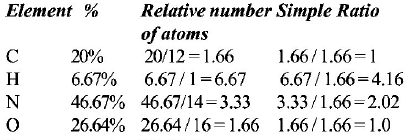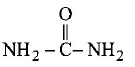101.
When the imidazole ring of Histidine is protonated, tendency of nitrogen to be protonated ( proton migrates from $$- COOH$$ ) is in the order

A
$$\beta > \gamma > \alpha $$
B
$$\gamma > \beta > \alpha $$
C
$$\gamma > \alpha > \beta $$
D
$$\beta > \alpha > \gamma $$
Answer :
$$\gamma > \alpha > \beta $$
102.
Identify $$P, Q, R, S, T$$ in the given sequence of reaction.

$$P$$
$$Q$$
$$R$$
$$S$$
$$T$$
(a)
$${C_6}{H_5}N{O_2}$$
$${C_6}{H_5}CN$$
$${C_6}{H_5}NC$$
$${C_6}{H_5}N{H_2}$$
$$C{H_3}COOH$$
(b)
$${C_6}{H_6}$$
$${C_6}{H_5}N{O_2}$$
$${C_6}{H_5}NC$$
$${C_6}{H_5}N{H_2}$$
$$C{H_3}COCl$$
(c)
$${C_6}{H_5}N{H_2}$$
$${C_6}{H_5}NHC{H_3}$$
$${C_6}{H_5}N{H_2}$$
$${C_6}{H_5}CN$$
$$C{H_3}Cl$$
(d)
$${C_6}{H_5}N{O_2}$$
$${C_6}{H_5}N{H_2}$$
$${C_6}{H_5}NC$$
$${C_6}{H_5}NHC{H_3}$$
$${\left( {C{H_3}CO} \right)_2}O$$
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(d)
103.
Which of the following should be most volatile?
$$\eqalign{
& \left( {\text{i}} \right)C{H_3}C{H_2}C{H_2}N{H_2} \cr
& \left( {{\text{ii}}} \right){\left( {C{H_3}} \right)_3}N \cr} $$
$$\left( {{\text{iii}}} \right)$$ 
$$\left( {{\text{iv}}} \right)C{H_3}C{H_2}C{H_3}$$
A
(ii)
B
(iv)
C
(i)
D
(iii)
Answer :
(iv)
104. The reaction of chloroform with alcoholic $$KOH$$ and $$p - $$toluidine forms
A


B


C


D


Answer :


105.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
\[C{H_3}N{H_2} + CHC{l_3} + KOH\xrightarrow{\Delta }\]
1.
$$C{H_3}N{H_2}$$
b.
$$C{H_3}CON{H_2} + B{r_2} + KOH \to $$
2.
$$C{H_3}OH$$
c.
$$C{H_3}N{H_2} + NaN{O_2} + HCl \to $$
3.
$$C{H_3}NHC{H_3}$$
d.
\[C{H_3}NC + 4H\xrightarrow{{Pt}}\]
4.
$$C{H_3}NC$$
A
a - 1, b - 2, c - 3, d - 4
B
a - 2, b - 3, c - 4, d - 1
C
a - 4, b - 1, c - 2, d - 3
D
a - 3, b - 4, c - 1, d - 2
Answer :
a - 4, b - 1, c - 2, d - 3
106. An organic compound having molecular mass 60 is found to contain $$C = 20\% ,H = 6.67\% \,{\text{and}}\,N = 46.67\% $$ while rest is oxygen. On heating it gives $$N{H_3}$$ alongwith a solid residue. The solid residue give violet colour with alkaline copper sulphate solution. The compound is
A
$$C{H_3}C{H_2}CON{H_2}$$
B
$${\left( {N{H_2}} \right)_2}CO$$
C
$$C{H_3}CON{H_2}$$
D
$$C{H_3}NCO$$
Answer :
$${\left( {N{H_2}} \right)_2}CO$$
107. Identify the incorrect IUPAC name.
A
$${\left( {C{H_3}C{H_2}} \right)_2}NC{H_3} - N$$ -Ethyl-$$N$$-methylethanamine
B
$${\left( {C{H_3}} \right)_3}CN{H_2} - 2$$ -Methylpropan-2-amine
C
$$C{H_3}NHCH{\left( {C{H_3}} \right)_2}$$ $$-N$$-Methylpropan-2-amine
D
$${\left( {C{H_3}} \right)_2}CHN{H_2} - 2,2$$ -Dimethyl-$$N$$-propanamine
Answer :
$${\left( {C{H_3}} \right)_2}CHN{H_2} - 2,2$$ -Dimethyl-$$N$$-propanamine
108. In Hofmann bromamide degradation, one of the important steps is the migration of
A
an alkyl group without its electron pair to electron deficient $$N$$ atom.
B
an alkyl group with its electron pair to electron deficient $$O$$ atom.
C
an alkyl group with its electron pair to electron rich $$N$$ atom.
D
an alkyl group with its electron pair to electron deficient $$N$$ atom.
Answer :
an alkyl group with its electron pair to electron deficient $$N$$ atom.
109. Which of the following is used as Hinsberg's reagent?
A
$${C_6}{H_5}S{O_2}Cl$$
B
$${C_6}{H_5}S{O_3}H$$
C
$${C_6}{H_5}NHC{H_3}$$
D
$${C_6}{H_5}COC{H_3}$$
Answer :
$${C_6}{H_5}S{O_2}Cl$$
110. Aniline dissolved in dilute $$HCl$$ is reacted with sodium nitrate at $${0^ \circ }C$$ . This solution was added dropwise to a solution containing equimolar mixture of aniline and phenol in dil. $$HCl.$$ The structure of the major product is:
A


B


C


D


Answer :