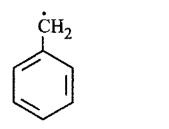
71.
The correct priorities for the substituents shown below, according to the $$E-Z$$ sequence rule is

A
II, III, V, I, IV
B
V, II, I, IV, III
C
III, IV, I, II, V
D
II, V, I, IV, III
Answer :
II, III, V, I, IV
73.
Among the following compounds the decreasing order of reactivity towards
electrophilic substitution is

A
II > I > III > IV
B
III > I > II > IV
C
IV > I > II > III
D
I > II > III > IV
Answer :
III > I > II > IV
74. How many $$\sigma $$ and $$\pi $$ bonds are present in $$C{H_2} = CH - CH = CH - C{H_3}?$$
A
$$9\sigma ,4\pi $$
B
$$12\sigma ,2\pi $$
C
$$12\sigma ,6\pi $$
D
$$10\sigma ,3\pi $$
Answer :
$$12\sigma ,2\pi $$
75. An organic compound gave $$0.4655\,g$$ of $$C{O_2}$$ on complete combustion. If the mass of the compound taken was $$0.2115\,g,$$ what is the percentage of $$C$$ in it?
A
$$13.30\% $$
B
$$26.67\% $$
C
$$60.03\% $$
D
$$28.80\% $$
Answer :
$$60.03\% $$
76. A sample of $$0.50\,g$$ of an organic compound was treated according to Kjeldahl's method. The ammonia evolved was absorbed in $$0.50\,mL$$ of $$0.5\,M\,{H_2}S{O_4}.$$ The residual acid required $$60\,mL$$ of $$0.5\,M$$ solution of $$NaOH$$ for neutralisation. What would be the percentage composition of nitrogen in the compound?
A
50
B
60
C
56
D
44
Answer :
56
77.
Given below is the developed chromatogram of a mixture of pigments.

$${R_f}$$ values for $$x$$ and $$y$$ can be expressed as
A
$$\frac{x}{z},\frac{y}{z}$$
B
$$\frac{x}{y},\frac{y}{z}$$
C
$$xz,yz$$
D
$$\frac{z}{x},\frac{z}{y}$$
Answer :
$$\frac{x}{z},\frac{y}{z}$$
78.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
Beilstein test
1.
Sulphur
b.
Sodium nitroprusside
2.
Carbon
c.
Liebig's method
3.
Nitrogen
d.
Kjeldahl's method
4.
Chlorine
A
a - 1, b - 2, c - 3, d - 4
B
a - 3, b - 2, c - 1, d - 4
C
a - 4, b - 1, c - 2, d - 3
D
a - 2, b - 3, c - 4, d - 1
Answer :
a - 4, b - 1, c - 2, d - 3
79. In the hydrocarbon $$\mathop {C{H_3}}\limits_6 - \mathop {CH}\limits_5 = \mathop {CH}\limits_4 - \mathop {C{H_2}}\limits_3 - \mathop C\limits_2 \equiv \mathop {CH}\limits_1 $$ the state of hybridisation of carbons 1, 3 and 5 are in the following sequence
A
$$s{p^2},sp,s{p^3}$$
B
$$sp,s{p^3},s{p^2}$$
C
$$sp,s{p^2},s{p^3}$$
D
$$s{p^3},s{p^2},sp$$
Answer :
$$sp,s{p^3},s{p^2}$$
80.
Arrange the following compounds in order of increasing
dipole moment.
Toluene (i)
$$m$$ - dichlorobenzene (ii)
$$o$$ - dichlorobenzene (iii)
$$p$$ - dichlorobenzene (iv)
A
(i) < (iv) < (ii) < (iii)
B
(iv) < (i) < (ii) < (iii)
C
(iv) < (i) < (iii) < (ii)
D
(iv) < (ii) < (i) < (iii)
Answer :
(iv) < (i) < (ii) < (iii)