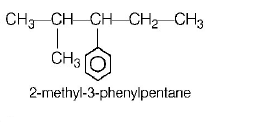
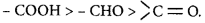101.
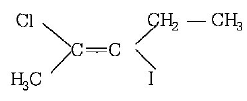
IUPAC name of the compound
 is
is
A
$$trans-3- iodo-4-chloro-3-pentene$$
B
$$cis-2-chloro-3-iodo-2-pentene$$
C
$$trans-2-chloro-3-iodo-2-pentene$$
D
$$cis-3-iodo-4-chloro-3-pentene$$
Answer :
$$trans-2-chloro-3-iodo-2-pentene$$
102.
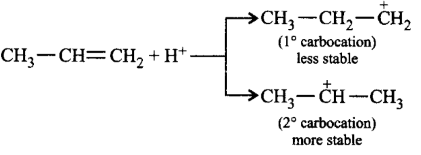
Electrophilic addition reactions proceed in two steps. The first step involves the addition of an electrophile. Name the type of intermediate formed in the first step of the following addition reaction.
$${H_3}C - HC = C{H_2} + {H^ + } \to ?$$
A
$${2^ \circ }$$ Carbanion
B
$${1^ \circ }$$ Carbocation
C
$${2^ \circ }$$ Carbocation
D
$${1^ \circ }$$ Carbanion
Answer :
$${2^ \circ }$$ Carbocation
103. Glycerine can be purified by
A
vacuum distillation
B
simple distillation
C
steam distillation
D
fractional distillation
Answer :
vacuum distillation
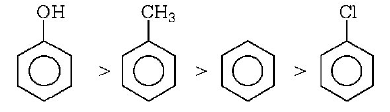
104. Which one of the following compounds will be most easily attacked by an electrophile?
A


B


C
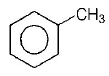

D
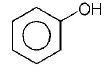

Answer :


105. The fragrance of flowers is due to the presence of some steam volatile organic compounds called essential oils. These are generally insoluble in water at room temperature but are miscible with water vapour in vapour phase. A suitable method for the extraction of these oils from the flowers is
A
distillation
B
crystallisation
C
distillation under reduced pressure
D
steam distillation
Answer :
steam distillation
106.
The radical  is aromatic because it has
is aromatic because it has
A
$$6p$$ - orbitals and 6 unpaired electrons
B
$$7p$$ - $$2$$ orbitals and 6 unpaired electrons
C
$$7p$$ - orbitals and 7 unpaired electrons
D
$$6p$$ - orbitals and 7 unpaired electrons
Answer :
$$7p$$ - orbitals and 7 unpaired electrons
107. The enol form of acetone after treatment with $${D_2}O$$ gives :
A
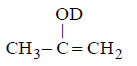
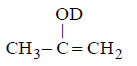
B


C


D


Answer :
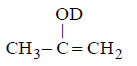
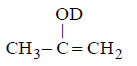
108.
Which of the following numberings is correct ?

A
$$A$$
B
$$B$$
C
$$C$$
D
$$D$$
Answer :
$$D$$
110.
The correct IUPAC name of the compound 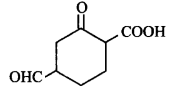 is
is
A
4-formyl-2-oxocyclohexanecarboxylic acid
B
4-carboxy-2-oxocyclohexanal
C
4-carboxy-1-formylcyclohexanone
D
2-carboxy- 5-formyl-1-oxocyclohexane
Answer :
4-formyl-2-oxocyclohexanecarboxylic acid

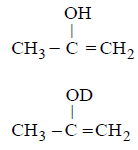 with $${D_2}O$$ it gives
with $${D_2}O$$ it gives