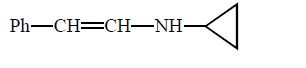171.
Predict the correct intermediate and product in the following reaction.
\[\begin{align}
& {{H}_{3}}C-C\equiv CH\xrightarrow[HgS{{O}_{4}}]{{{H}_{2}}O,{{H}_{2}}S{{O}_{4}}} \\
& \underset{\left( A \right)}{\mathop{\text{Intermediate}}}\,\to \underset{\left( B \right)}{\mathop{\text{Product}}}\, \\
\end{align}\]
A
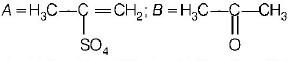
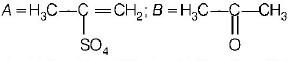
B
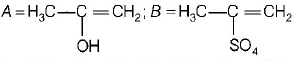
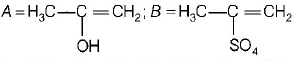
C
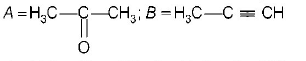
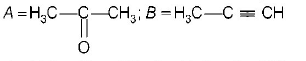
D


Answer :


172. The compound that will not give iodoform on treatment with alkali and iodine is :
A
acetone
B
ethanol
C
diethyl ketone
D
isopropyl alcohol
Answer :
diethyl ketone
173. Which of the following statements is incorrect?
A
$$FeC{l_3}$$ is used in the detection of phenols.
B
Fehling solution is used in the detection of glucose.
C
Tollens' reagent is used in the detection of unsaturation.
D
$$NaHS{O_3}$$ is used in the detection of carbonyl compounds.
Answer :
Tollens' reagent is used in the detection of unsaturation.
174. Formalin is an aqueous solution of
A
fluorescein
B
formic acid
C
formaldehyde
D
furfuraldehyde
Answer :
formaldehyde
175.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
Clemmensen reduction
1.
$${\text{Conc}}{\text{.}}\,KOH$$
b.
Rosenmund reduction
2.
$$Zn/Hg + {\text{conc}}.\,HCl$$
c.
Iodoform reaction
3.
$${H_2}/Pd{\text{ - }}BaS{O_4}$$
d.
Cannizzaro reaction
4.
$$NaOH + {I_2}$$
A
a - 1, b - 3, c - 2, d - 4
B
a - 3, b - 4, c - 1, d - 2
C
a - 2, b - 3, c - 4, d - 1
D
a - 4, b - 1, c - 2, d - 3
Answer :
a - 2, b - 3, c - 4, d - 1
176. The reaction products of \[{{C}_{6}}{{H}_{5}}OC{{H}_{3}}+HI\xrightarrow{\Delta }\] is :
A
\[{{C}_{6}}{{H}_{5}}OH+C{{H}_{3}}I\]
B
\[{{C}_{6}}{{H}_{5}}I+C{{H}_{3}}OH\]
C
\[{{C}_{6}}{{H}_{5}}C{{H}_{3}}+HOI\]
D
\[{{C}_{6}}{{H}_{6}}+C{{H}_{3}}OH\]
Answer :
\[{{C}_{6}}{{H}_{5}}OH+C{{H}_{3}}I\]
178.
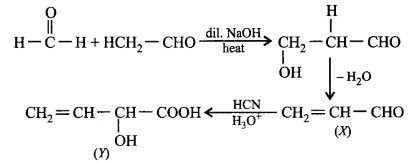
Study the following sequence of reactions and identify the product $$(Y).$$
\[C{{H}_{3}}CHO+HCHO\xrightarrow[\text{heat}]{\text{dil}\text{.}\,NaOH}\] \[X\xrightarrow[{{H}_{3}}{{O}^{+}}]{HCN}Y\]
A
\[C{{H}_{2}}=CH\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,COOH\]
B
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,OH
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,CN \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,COOH\]
C
\[C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\,\, \\
OH\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,COOH\]
D
\[C{{H}_{2}}=CH\underset{\begin{smallmatrix}
|\,\,\,\,\,\, \\
CN\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,COOH\]
Answer :
\[C{{H}_{2}}=CH\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,COOH\]
179. The oxidation of benzene by $${V_2}{O_5}$$ in the presence of air produces
A
benzoic anhydride
B
maleic anhydride
C
benzoic acid
D
benzaldehyde
Answer :
maleic anhydride
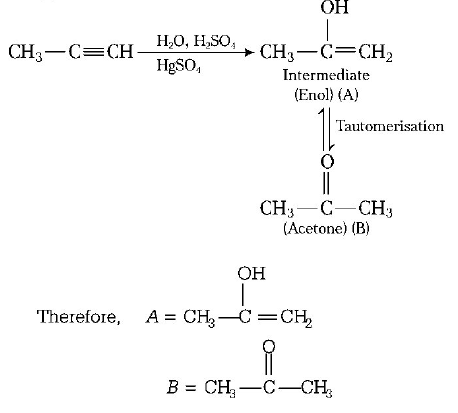
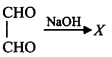
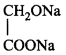
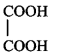
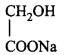
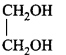

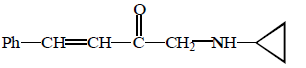
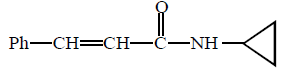
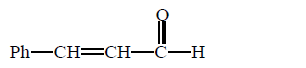
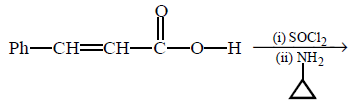 \[\left( A \right);\] Product $$(A)$$ of the reaction is :
\[\left( A \right);\] Product $$(A)$$ of the reaction is :