111. Which one of the following is reduced with zinc and hydrochloric acid to give the corresponding hydrocarbon?
A
Acetamide
B
Acetic acid
C
Ethyl acetate
D
Butan - 2 - one
Answer :
Butan - 2 - one
112. In the following reaction, product $$(P)$$ is \[R\overset{\begin{smallmatrix} O \\ \parallel \end{smallmatrix}}{\mathop{-C-}}\,Cl\xrightarrow[\frac{Pd}{BaS{{O}_{4}}}]{{{H}_{2}}}P\]
A
$$RCHO$$
B
$$RC{H_3}$$
C
$$RCOOH$$
D
$$RC{H_2}OH$$
Answer :
$$RCHO$$
113.
The reaction,
\[C{{H}_{3}}CHO\xrightarrow{Zn\left( Hg \right)/Conc.\,HCl\left[ H \right]}C{{H}_{3}}C{{H}_{3}}\] is
A
Cannizaro’s reaction
B
Rosenmund reduction
C
Wolf-Kishner reduction
D
Clemmenson reduction
Answer :
Clemmenson reduction
114. Which of the following aldehydes will show Cannizzaro reaction?
A
$$HCHO$$
B
$${C_6}{H_5}CHO$$
C
$${\left( {C{H_3}} \right)_3}CCHO$$
D
$${\text{All of these}}$$
Answer :
$${\text{All of these}}$$
115. The reagent which does not react with both, acetone and benzaldehyde is ________.
A
sodium hydrogensulphite
B
phenyl hydrazine
C
Fehling's solution
D
Grignard reagent
Answer :
Fehling's solution
116. $$C{H_3}CHO$$ and $${C_6}{H_5}C{H_2}CHO$$ can be distinguished chemically by
A
Benedict test
B
iodoform test
C
Tollen’s reagent test
D
Fehling solution test
Answer :
iodoform test
117.
The organic product formed in the reaction
\[{{C}_{6}}{{H}_{5}}COOH\xrightarrow[II\,{{H}_{3}}{{O}^{+}}]{I\,LiAl{{H}_{4}}}\]
A
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH\]
B
\[{{C}_{6}}{{H}_{5}}COOH\,\And C{{H}_{4}}\]
C
\[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\,\And \,C{{H}_{3}}OH\]
D
\[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\,\And \,C{{H}_{4}}\]
Answer :
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH\]
118. The product formed by the reaction of an aldehyde with a primary amine is
A
Ketone
B
Carboxylic acid
C
Aromatic acid
D
Schiff base
Answer :
Schiff base
119. Acetophenone when reacted with a base, $${C_2}{H_5}ONa,$$ yields a stable compound which has the structure
A


B


C


D


Answer :


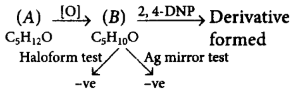
120. A compound $$'A'$$ having the molecular formula $${C_5}{H_{12}}O,$$ on oxidation gives a compound $$'B'$$ with molecular formula $${C_5}{H_{10}}O.$$ Compound $$'B'$$ gave a 2, 4-dinitrophenylhydrazine derivative but did not answer haloform test or silver mirror test. The structure of compound $$'A'$$ is
A
$$C{H_3} - C{H_2} - C{H_2} - C{H_2} - C{H_2}$$ $$ - OH$$
B
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}}\]
C
\[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}-C{{H}_{3}}\]
D
\[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
C{{H}_{3}}\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}-OH\]
Answer :
\[C{{H}_{3}}-C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}-C{{H}_{3}}\]

 group
reacts with $$NaOH$$ and $${I_2}$$ to give yellow crystals of iodoform while $${C_6}{H_5}C{H_2}CHO$$ does not react with it.
group
reacts with $$NaOH$$ and $${I_2}$$ to give yellow crystals of iodoform while $${C_6}{H_5}C{H_2}CHO$$ does not react with it. 


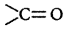 group.
group.