371. Which of the following statements is not correct?
A
All the oxides of halogens are powerful oxidants.
B
The compounds of oxygen and fluorine are not called oxides but fluorides.
C
Fluorine forms oxoacids.
D
In oxyhalides, bonds are mainly covalent due to small difference in electronegativity of oxygen and halogens.
Answer :
Fluorine forms oxoacids.
372. A gas that cannot be collected over water is :
A
$$\,{N_2}$$
B
$${O_2}$$
C
$$S{O_2}\,$$
D
$$P{H_3}\,$$
Answer :
$$S{O_2}\,$$
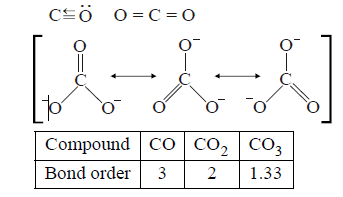
373. The correct order of increasing $$C - O$$ bond length of $$CO,C{O_2}$$ and $$CO_3^{2 - }$$ is :
A
$$CO_3^{2 - } < C{O_2} < CO$$
B
$$C{O_2} < CO_3^{2 - } < CO$$
C
$$CO < CO_3^{2 - } < C{O_2}$$
D
$$CO < C{O_2} < CO_3^{2 - }$$
Answer :
$$CO < C{O_2} < CO_3^{2 - }$$
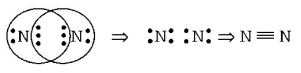
374. Number of electrons shared in the formation of nitrogen molecule is
A
6
B
10
C
2
D
8
Answer :
6
375. Which of the following does not depict properties of fullerenes?
A
Fullerenes are made by heating graphite.
B
Fullerenes are pure forms of carbon.
C
Fullerenes have open cage structure like ice.
D
$${C_{60}}$$ is called Buckminsterfullerene.
Answer :
Fullerenes have open cage structure like ice.
376.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
Laughing gas
1.
Hydrazoic acid
b.
Anhydride of $$HN{O_3}$$
2.
Nitrous oxide
c.
Anhydride of $$HP{O_3}$$
3.
Nitrogen pentoxide
d.
Acid hydride of nitrogen
4.
Phosphorus pentoxide
A
a - 1, b - 2, c - 3, d - 4
B
a - 4, b - 1, c - 2, d - 3
C
a - 2, b - 3, c - 4, d - 1
D
a - 3, b - 4, c - 1, d - 2
Answer :
a - 2, b - 3, c - 4, d - 1
377.
Match the compounds given in column I with the hybridisation and shape given in Column II and mark the correct option.
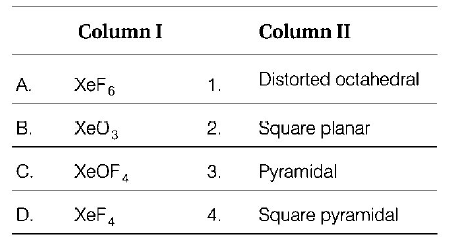
Codes
$$\eqalign{
& \,\,\,\,\,\,\,\,\,\,\,{\text{A}}\,\,\,\,\,{\text{B}}\,\,\,\,\,{\text{C}}\,\,\,\,\,{\text{D}} \cr
& \left( {\text{a}} \right)\,\,\,1\,\,\,\,\,\,\,{\text{2}}\,\,\,\,\,\,{\text{4}}\,\,\,\,\,\,{\text{3}} \cr
& \left( {\text{b}} \right)\,\,{\text{4}}\,\,\,\,\,\,\,\,{\text{3}}\,\,\,\,\,\,{\text{1}}\,\,\,\,\,\,{\text{2}} \cr
& \left( {\text{c}} \right)\,\,{\text{4}}\,\,\,\,\,\,\,\,{\text{1}}\,\,\,\,\,\,{\text{2}}\,\,\,\,\,\,{\text{3}} \cr
& \left( {\text{d}} \right)\,\,{\text{1}}\,\,\,\,\,\,\,\,{\text{3}}\,\,\,\,\,\,{\text{4}}\,\,\,\,\,\,{\text{2}} \cr} $$
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(d)
378. Decreasing order of stability of $${O_2},O_2^ - ,O_2^ + $$ and $$O_2^{2 - }$$ is
A
$$O_2^ + > {O_2} > O_2^ - > O_2^{2 - }$$
B
$$O_2^{2 - } > O_2^ - > {O_2} > O_2^ + $$
C
$${O_2} > O_2^ + > O_2^{2 - } > O_2^ - $$
D
$$O_2^ - > O_2^{2 - } > O_2^ + > {O_2}$$
Answer :
$$O_2^ + > {O_2} > O_2^ - > O_2^{2 - }$$
379. Example of a three-dimensional silicate is :
A
Zeolites
B
Ultramarines
C
Feldspars
D
Beryls
Answer :
Feldspars
380.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
Borax
1.
$$N{a_3}Al{F_6}$$
b.
Inorganic benzene
2.
$$N{a_2}{B_4}{O_7} \cdot 10{H_2}O$$
c.
Cryolite
3.
$$A{l_2}{O_3} \cdot 2{H_2}O$$
d.
Bauxite
4.
$${B_3}{N_3}{H_6}$$
A
a - 2, b - 4, c - 1, d - 3
B
a - 1, b - 2, c - 3, d - 4
C
a - 2, b - 3, c - 1, d - 4
D
a - 3, b - 1, c - 2, d - 4
Answer :
a - 2, b - 4, c - 1, d - 3