361. The oxidation state of central atom in the anion of compound $$Na{H_2}P{O_2}$$ will be
A
+ 3
B
+ 5
C
+ 1
D
- 3
Answer :
+ 1
362. Which of the following statements is correct?
A
Group-13 elements show only one oxidation state i.e., + 3.
B
$$Tl\left( {{\text{III}}} \right)$$ salts undergo disproportionation.
C
$$Si{O_2}$$ is covalent solid.
D
$$B{\left( {OMe} \right)_3}$$ imparts violet colour to Bunsen flame.
Answer :
$$Si{O_2}$$ is covalent solid.
363. Which of the following is not tetrahedral in shape?
A
$$NH_4^ + $$
B
$$SiC{l_4}$$
C
$$S{F_4}$$
D
$$SO_4^{2 - }$$
Answer :
$$S{F_4}$$
364. Which of the following is used in the preparation of chlorine?
A
Only $$Mn{O_2}$$
B
Only $$KMn{O_4}$$
C
Both $$Mn{O_2}$$ and $$KMn{O_4}$$
D
Either $$Mn{O_2}$$ and $$KMn{O_4}$$
Answer :
Both $$Mn{O_2}$$ and $$KMn{O_4}$$
365. Which statement is not true about potash alum ?
A
On heating it melts and loses its water of crystallization.
B
It’s aqueous solution is basic in nature.
C
It is used in dyeing industries.
D
It’s empirical formula is $$KAl{\left( {S{O_4}} \right)_2}.12{H_2}O.$$
Answer :
It’s aqueous solution is basic in nature.
366. Which of the following is not oxidized $${O_3}?$$
A
$$Kl$$
B
$$FeS{O_4}$$
C
$$KMn{O_4}$$
D
$${K_2}Mn{O_4}$$
Answer :
$$KMn{O_4}$$
367. The decreasing order of power of boron halides to act as Lewis acids is
A
$$B{F_3} > BC{l_3} > BB{r_3}$$
B
$$BB{r_3} > BC{l_3} > B{F_3}$$
C
$$BC{l_3} > B{F_3} > BB{r_3}$$
D
$$BC{l_3} > BB{r_3} > B{F_3}$$
Answer :
$$BB{r_3} > BC{l_3} > B{F_3}$$
368. Why is sulphur dioxide considered as an air pollutant?
A
It increases the temperature of the atmosphere.
B
It is used as insecticide which causes air pollution.
C
It causes acid rain due to formation of sulphuric acid on combining with $${O_2}$$ and $${H_2}O.$$
D
It is a strong oxidising agent hence oxidises the other components of air.
Answer :
It causes acid rain due to formation of sulphuric acid on combining with $${O_2}$$ and $${H_2}O.$$
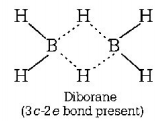
369. Which of the following compound has a 3-centre bond?
A
Diborane
B
$$C{O_2}$$
C
Boron trifluoride
D
Ammonia
Answer :
Diborane
370. In the clathrates of xenon with water, the nature of bonding between xenon and water molecule is
A
covalent
B
hydrogen bonding
C
co-ordinate
D
dipole-induced dipole interaction
Answer :
dipole-induced dipole interaction