331. One mole of fluorine is reacted with two moles of hot and concentrated $$KOH.$$ The products formed are $$KF,{H_2}O$$ and $${O_2}.$$ The molar ratio of $$KF,{H_2}O$$ and $${O_2}$$ respectively is
A
1 : 1 : 2
B
2 : 1 : 0.5
C
1 : 2 : 1
D
2 : 1 : 2
Answer :
2 : 1 : 0.5
332. Which one of the following does not have a pyramidal shape ?
A
$${\left( {C{H_3}} \right)_3}N$$
B
$${\left( {Si{H_3}} \right)_3}N$$
C
$$P{\left( {C{H_3}} \right)_3}$$
D
$$P{\left( {Si{H_3}} \right)_3}$$
Answer :
$${\left( {Si{H_3}} \right)_3}N$$
333. Which of the following reactions is an example of a redox reaction?
A
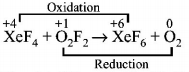
$$Xe{F_4} + {O_2}{F_2} \to Xe{F_6} + {O_2}$$
B
$$Xe{F_2} + P{F_5} \to {\left[ {XeF} \right]^ + }PF_6^ - $$
C
$$Xe{F_6} + {H_2}O \to XeO{F_4} + 2HF$$
D
$$Xe{F_6} + 2{H_2}O \to Xe{O_2}{F_2} + 4HF$$
Answer :
$$Xe{F_4} + {O_2}{F_2} \to Xe{F_6} + {O_2}$$
334. An element $$\left( X \right)$$ forms compounds of the formula $$XC{l_3},{X_2}{O_5}$$ and $$C{a_3}{X_2}$$ but does not form $$XC{l_5}.$$ Which of the following is the element $$X?$$
A
$$B$$
B
$$Al$$
C
$$N$$
D
$$P$$
Answer :
$$N$$
335. $${P_4}{O_{10}}$$ is not used to dry $$N{H_3}$$ gas because
A
$${P_4}{O_{10}}$$ reacts with moisture in $$N{H_3}.$$
B
$${P_4}{O_{10}}$$ is not a drying agent.
C
$${P_4}{O_{10}}$$ is acidic and $$N{H_3}$$ is basic.
D
$${P_4}{O_{10}}$$ is basic and $$N{H_3}$$ is acidic.
Answer :
$${P_4}{O_{10}}$$ is acidic and $$N{H_3}$$ is basic.
336.
$$\eqalign{
& A + {H_2}O \to B + HCl \cr
& B + {H_2}O \to C + HCl \cr} $$
Compound $$(A), (B)$$ and $$(C)$$ will be respectively :
A
$$PC{l_5},POC{l_3},{H_3}P{O_3}$$
B
$$PC{l_5},POC{l_3},{H_3}P{O_4}$$
C
$$SOC{l_2},POC{l_3},{H_3}P{O_3}$$
D
$$PC{l_3},POC{l_3},{H_3}P{O_4}$$
Answer :
$$PC{l_5},POC{l_3},{H_3}P{O_4}$$
337. The element which exists in liquid state for a wide range of temperature and can be used for measuring high temperature is
A
$$B$$
B
$$Al$$
C
$$Ga$$
D
$$In$$
Answer :
$$Ga$$
338.
Borax is converted into crystalline boron by the following steps :
\[\text{Borax}\xrightarrow{X}{{H}_{3}}B{{O}_{3}}\xrightarrow{\Delta }{{B}_{2}}{{O}_{3}}\xrightarrow[\Delta ]{Y}B\]
$$X$$ and $$Y$$ are respectively :
A
$$HCl,Mg$$
B
$$HCl,C$$
C
$$C,Al$$
D
$$HCl,Al$$
Answer :
$$HCl,Al$$
339. In the manufacture of bromine from sea water the mother liquor containing bromide is treated with
A
carbon dioxide
B
chlorine
C
iodine
D
sulphur dioxide
Answer :
chlorine
340. The stability of dihalides of $$Si,Ge,Sn$$ and $$Pb$$ increases steadily in the sequence
A
$$Pb{X_2} < Sn{X_2} < Ge{X_2} < Si{X_2}$$
B
$$Ge{X_2} < Si{X_2} < Sn{X_2} < Pb{X_2}$$
C
$$Si{X_2} < Ge{X_2} < Pb{X_2} < Sn{X_2}$$
D
$$Si{X_2} < Ge{X_2} < Sn{X_2} < Pb{X_2}$$
Answer :
$$Si{X_2} < Ge{X_2} < Sn{X_2} < Pb{X_2}$$