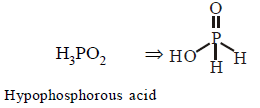281. What happens when a mixture of cobalt oxide and borax is heated in a flame on a loop of platinum wire?
A
A transparent white bead is formed.
B
A bright pink coloured $$NaB{O_2}$$ bead is formed.
C
A blue coloured $$Co{\left( {B{O_2}} \right)_2}$$ bead is formed.
D
A red coloured $$Co{\left( {B{O_2}} \right)_2}$$ bead is formed.
Answer :
A blue coloured $$Co{\left( {B{O_2}} \right)_2}$$ bead is formed.
282. Which is the most thermodynamically stable allotropic form of phosphorus?
A
red
B
white
C
black
D
yellow
Answer :
black
283. Which of the following pairs is not correctly matched?
A
Allotropic form of sulphur which is more stable at room temperature - Rhombic
B
The hydride of group 16 which is liquid at room temperature - Water
C
The gas formed in the upper layers of atmosphere by action of $$UV$$ radiations - Nitrogen
D
The catalyst used in the manufacture of $${H_2}S{O_4}$$ by contact process - Vanadium pentoxide
Answer :
The gas formed in the upper layers of atmosphere by action of $$UV$$ radiations - Nitrogen
284. Which ordering of compounds is according to the decreasing order of the oxidation state of nitrogen?
A
$$HN{O_3},NO,N{H_4}Cl,{N_2}$$
B
$$\,HN{O_3},NO,{N_2},N{H_4}Cl$$
C
$$HN{O_3},N{H_4}Cl,NO,{N_2}$$
D
$$NO,HN{O_3},N{H_4}Cl,{N_2}$$
Answer :
$$\,HN{O_3},NO,{N_2},N{H_4}Cl$$
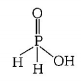
285. Strong reducing behaviour of $${H_3}P{O_2}$$ is due to
A
presence of one $$—OH$$ group and two $$P—H$$ bonds
B
high electron gain enthalpy of phosphorus
C
high oxidation state of phosphorus
D
presence of two $$—OH$$ groups and one $$P—H$$ bond
Answer :
presence of one $$—OH$$ group and two $$P—H$$ bonds
286. Which among the following is paramagnetic?
A
$$C{l_2}O$$
B
$$Cl{O_2}$$
C
$$C{l_2}{O_7}$$
D
$$C{l_2}{O_6}$$
Answer :
$$Cl{O_2}$$
287. Which of the following oxy-acids has the maximum number of hydrogens directly attached to phosphorus ?
A
$${H_4}{P_2}{O_7}$$
B
$${H_3}P{O_2}$$
C
$${H_3}P{O_3}$$
D
$${H_3}P{O_4}$$
Answer :
$${H_3}P{O_2}$$
288. When steam reacts with red hot coke to form $$C{O_2}$$ and hydrogen :
A
Water acts as an oxidising agent.
B
Water acts as a reducing agent.
C
Carbon acts as an oxidising agent.
D
There is no oxidation or reduction.
Answer :
Water acts as an oxidising agent.
289. On heating ammonium dichromate, the gas evolved is
A
oxygen
B
ammonia
C
nitrous oxide
D
nitrogen
Answer :
nitrogen
290. Pick out the wrong statement.
A
Nitrogen has the ability to form $$p\pi - p\pi $$ bonds with itself.
B
Bismuth forms metallic bonds in elemental state.
C
Catenation tendency is higher in nitrogen when compared with other elements of the same group.
D
Nitrogen has higher first ionisation enthalpy when compared with other elements of the same group.
Answer :
Catenation tendency is higher in nitrogen when compared with other elements of the same group.