121. Which of the following is laboratory preparation of dihydrogen?
A
$$3Fe + 4{H_2}O\left( {{\text{steam}}} \right) \to F{e_3}{O_4} + 4{H_2}$$
B
$$2Na + 2{H_2}O \to 2NaOH + {H_2}$$
C
$$Ca{H_2} + 2{H_2}O \to Ca{\left( {OH} \right)_2} + 2{H_2}$$
D
$$Zn + {H_2}S{O_4}\left( {{\text{dil}}{\text{.}}} \right) \to ZnS{O_4} + {H_2}$$
Answer :
$$Zn + {H_2}S{O_4}\left( {{\text{dil}}{\text{.}}} \right) \to ZnS{O_4} + {H_2}$$
122. Calgon used as a water softener is
A
$$N{a_2}\left[ {N{a_4}{{\left( {P{O_3}} \right)}_6}} \right]$$
B
$$N{a_4}\left[ {N{a_2}{{\left( {P{O_3}} \right)}_6}} \right]$$
C
$$N{a_4}\left[ {N{a_4}{{\left( {P{O_4}} \right)}_5}} \right]$$
D
$$N{a_4}\left[ {N{a_2}{{\left( {P{O_4}} \right)}_6}} \right]$$
Answer :
$$N{a_2}\left[ {N{a_4}{{\left( {P{O_3}} \right)}_6}} \right]$$
123.
The various types of hydrides and examples of each type are given below :
Hydride type
Compound
a.
Electron deficient
1.
$$LiH$$
b.
Saline
2.
$$C{H_4}$$
c.
Electron-precise
3.
$$N{H_3}$$
d.
Interstitial
4.
$${B_2}{H_6}$$
e.
Electron rich
5.
$$CrH$$
Choose the correct matching from the codes given below :
A
a - 2, b - 4, c - 5, d - 3, e - 1
B
a - 4, b - 1, c - 2, d - 5, e - 3
C
a - 4, b - 3, c - 5, d - 2, e - 1
D
a - 5, b - 3, c - 4, d - 2, e - 1
Answer :
a - 4, b - 1, c - 2, d - 5, e - 3
124. In group 6, only one metal forms hydride. This metal is
A
$$Mo$$
B
$$W$$
C
$$Cr$$
D
$$Sg$$
Answer :
$$Cr$$
125. Which of the following is a true structure of $${H_2}{O_2}$$ in solid phase?
A


B
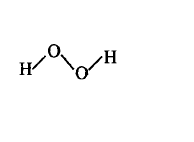

C
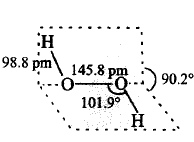

D
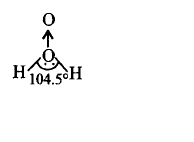

Answer :


126. Which of the following is not a process of preparation of hydrogen peroxide?
A
Auto - oxidation of 2 - ethylanthraquinol.
B
By passing oxygen through boiling water.
C
By oxidation of isopropyl alcohol.
D
By reaction of barium peroxide with dil. $${H_2}S{O_4}.$$
Answer :
By passing oxygen through boiling water.
127. Why does $${H^ + }$$ ion always get associated with other atoms or molecules?
A
Ionisation enthalpy of hydrogen resembles that of alkali metals.
B
Its reactivity is similar to halogens.
C
It resembles both alkali metals and halogens.
D
Loss of an electron from hydrogen atom results in a nucleus of very small size as compared to other atoms or ions. Due to small size it cannot exist free.
Answer :
Loss of an electron from hydrogen atom results in a nucleus of very small size as compared to other atoms or ions. Due to small size it cannot exist free.
128. Alkenes combine with carbon monoxide and hydrogen in presence of octacarbonyldicobalt as catalyst under high temperature and pressure to form
A
aldehydes which can be further reduced to alcohols by hydrogen
B
alkanes which are formed by addition of hydrogen
C
alcohols formed by reaction of $$CO$$ and hydrogen
D
ketones which can be further reduced to aldehydes by hydrogen
Answer :
aldehydes which can be further reduced to alcohols by hydrogen
129.
Mark the following statements as true or false.
(i) Ordinary hydrogen is a mixture of 75% $$ortho$$ and 25% $$para$$ - forms.
(ii) All the four atoms of molecule of $${H_2}{O_2}$$ lie in the same plane.
(iii) Hydrogen peroxide is neutral like water.
(iv) $${H_2}{O_2}$$ can be prepared from $$Ba{O_2}$$ but not from $$Mn{O_2}$$ and $$Pb{O_2}.$$
A
(i) and (iv) - true, (ii) and (iii) - false
B
(i) and (ii) - true, (iii) and (iv) - false
C
(iii) and (iv) - true, (i) and (ii) - false
D
(i) and (iii) - true, (ii) and (iv) - false
Answer :
(i) and (iv) - true, (ii) and (iii) - false
130.
Which property of hydrogen is shown by the following reactions?
\[\begin{align}
& \left( \text{i} \right)F{{e}_{3}}{{O}_{4}}+4{{H}_{2}}\to 3Fe+4{{H}_{2}}O \\
& \left( \text{ii} \right)CO+{{H}_{2}}\xrightarrow[C{{r}_{2}}{{O}_{3}}]{ZnO,}C{{H}_{3}}OH \\
\end{align}\]
A
Reducing character
B
Oxidising character
C
Combustibility
D
High reactivity
Answer :
Reducing character