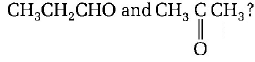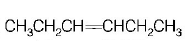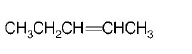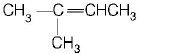81. Although benzene is highly unsaturated it does not undergo addition reactions. The explanation of this can be suggested as
A
$$\pi $$ - electrons of benzene ring are delocalised
B
since $$\pi $$ - electrons are present inside the ring, addition cannot take place
C
cyclic structures do not show addition reactions
D
benzene is not a reactive compound
Answer :
$$\pi $$ - electrons of benzene ring are delocalised
82. Identify the reagent from the following list which can easily distinguish between 1-butyne and 2-butyne
A
bromine, $$CC{l_4}$$
B
$${H_2},$$ Lindlar catalyst
C
dilute $${H_2}S{O_4},HgS{O_4}$$
D
ammonical $$C{u_2}C{l_2}$$ solution
Answer :
ammonical $$C{u_2}C{l_2}$$ solution
83. Which of the following reactions of methane is incomplete combustion?
A
\[2C{{H}_{4}}+{{O}_{2}}\xrightarrow{Cu/523\,K/100\,atm}2C{{H}_{3}}OH\]
B
\[C{{H}_{4}}+{{O}_{2}}\xrightarrow{M{{o}_{2}}{{O}_{3}}}HCHO+{{H}_{2}}O\]
C
$$C{H_4} + {O_2} \to {C_{\left( s \right)}} + 2{H_2}{O_{\left( l \right)}}$$
D
$$C{H_4} + 2{O_2} \to C{O_{2\left( g \right)}} + 2{H_2}{O_{\left( l \right)}}$$
Answer :
$$C{H_4} + {O_2} \to {C_{\left( s \right)}} + 2{H_2}{O_{\left( l \right)}}$$
84. Which of the following reactions is expected to readily give a hydrocarbon product in good yields ?
A
\[RCOOK\xrightarrow[\text{oxidation}]{\text{Electrolytic}}\]
B
\[RCOOAg\xrightarrow{{{l}_{2}}}\]
C
\[C{{H}_{3}}C{{H}_{3}}\xrightarrow[h\nu .]{C{{l}_{2}}}\]
D
\[{{\left( C{{H}_{3}} \right)}_{2}}CCl\xrightarrow{{{C}_{2}}{{H}_{5}}OH}\]
Answer :
\[RCOOK\xrightarrow[\text{oxidation}]{\text{Electrolytic}}\]
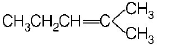
86.
Isomers of hexane, based on their branching, can be divided
into three distinct classes as shown in the figure.
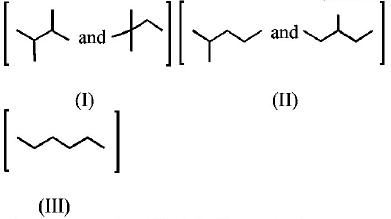
The correct order of their boiling point is
A
I > II > III
B
III > II > I
C
II > III > I
D
III > I > II
Answer :
III > II > I
87.
Which of the following will exhibit aromatic character?
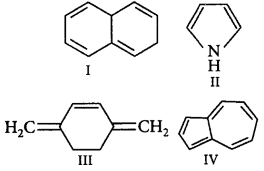
A
I, III
B
III, IV
C
II, IV
D
II, III
Answer :
II, IV
88.
The two compounds $$A$$ and $$B$$ obtained from 1-butyne can be distinguished by
\[B\xleftarrow[\left( ii \right)\,{{H}_{2}}{{O}_{2}}/O{{H}^{-}}]{\left( i \right)\,B{{H}_{3}}}C{{H}_{3}}C{{H}_{2}}C\equiv CH\xrightarrow{{{H}^{+}}/H{{g}^{2+}}}A\]
A
$$NaHS{O_3}$$
B
$${\text{litmus solution}}$$
C
$${\text{iodoform test}}$$
D
$$2,4{\text{ - }}DNP$$
Answer :
$${\text{iodoform test}}$$
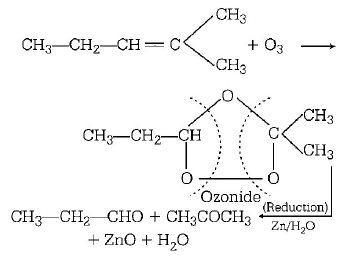
89.
In the following sequence of reactions, the alkene affords region 3
the compound $$'B’$$
\[C{{H}_{3}}-CH=CH-C{{H}_{3}}\xrightarrow{{{O}_{3}}}A\xrightarrow[Zn]{{{H}_{2}}O}B.\]
The compound $$B$$ is
A
$$C{H_3}C{H_2}CHO$$
B
$$C{H_3}COC{H_3}$$
C
$$C{H_3}C{H_2}COC{H_3}$$
D
$$C{H_3}CHO$$
Answer :
$$C{H_3}CHO$$
90.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
Alkyl halide + Sodium in presence of dry ether
1.
Sulphonation
b.
Arene + Acid halide in presence of $$AlC{l_3}$$
2.
Wurtz reaction
c.
Arene + Fuming sulphuric acid
3.
Catalytic hydrogenation
d.
Arene + Hydrogen in presence of $$Ni$$
4.
Friedel-Crafts reaction
A
a - 1, b - 3, c - 2, d - 4
B
a - 4, b - 2, c - 3, d - 1
C
a - 3, b - 1, c - 4, d - 2
D
a - 2, b - 4, c - 1, d - 3
Answer :
a - 2, b - 4, c - 1, d - 3