121. Which one of the following has the shortest carbon-carbon bond length?
A
Benzene
B
Ethene
C
Ethyne
D
Ethane
Answer :
Ethyne
122. The reaction of propene with $$HOCl\left( {C{l_2} + {H_2}O} \right)$$ proceeds through the intermediate :
A
$$C{H_3} - CH\left( {OH} \right) - CH_2^ + $$
B
$$C{H_3} - CHCl - CH_2^ + $$
C
$$C{H_3} - C{H^ + } - C{H_2} - OH$$
D
$$C{H_3} - C{H^ + } - C{H_2} - Cl$$
Answer :
$$C{H_3} - C{H^ + } - C{H_2} - Cl$$
124. Which is the correct symbol relating the hetero Kekule structure of benzene?
A
$$ \rightleftharpoons $$
B
$$ \to $$
C
$$ \equiv $$
D
$$ \leftrightarrow $$
Answer :
$$ \leftrightarrow $$
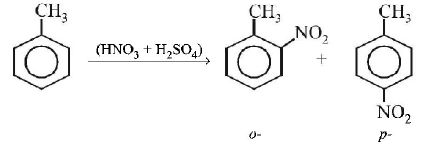
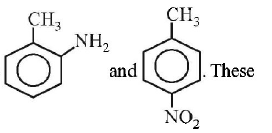
125. Toluene is nitrated and the resulting product is reduced with tin and hydrochloric acid. The product so obtained is diazotised and then heated wth cuprous bromide. The reaction mixture so formed contans
A
mixture of $$o - $$ and $$p - $$ bromotoluenes
B
mixture of $$o - $$ and $$p - $$ dibromobenzenes
C
mixture of $$o - $$ and $$p - $$ bromoanilines
D
mixture of $$o - $$ and $$m - $$ bromotoluenes
Answer :
mixture of $$o - $$ and $$p - $$ bromotoluenes
126. Propyne and propene can be distinguished by
A
$$conc.\,\,{H_2}S{O_4}$$
B
$$B{r_2}\,{\text{in}}\,CC{l_4}$$
C
$$dil.\,KMn{O_4}$$
D
$$AgN{O_3}$$ in ammonia
Answer :
$$AgN{O_3}$$ in ammonia
127. The major constituent of natural gas is
A
methane
B
propane
C
butane
D
hexane
Answer :
methane
128. One mole of 1, 2-dibromopropane on treatment with $$X$$ moles of $$NaN{H_2}$$ followed by treatment with ethyl bromide gave 2-pentyne. The value of $$X$$ is
A
one
B
two
C
three
D
four
Answer :
three
129.
Consider the following compounds :

the correct order towards electrophilic substitution reaction is
A
(iv) > (iii) > (ii) > (i)
B
(i) > (ii) > (iii) > (iv)
C
(iv) > (iii) > (i) > (ii)
D
(iii) > (iv) > (i) > (ii)
Answer :
(iii) > (iv) > (i) > (ii)
130.
Similar to alkenes and alkynes benzene also undergoes ozonolysis. In the sequence of the given reaction identify $$X$$ and $$Y.$$
 \[+\,{{O}_{3}}\to X\xrightarrow{\frac{Zn}{{{H}_{2}}O}}Y\]
\[+\,{{O}_{3}}\to X\xrightarrow{\frac{Zn}{{{H}_{2}}O}}Y\]
A
$$X =$$ Triozonide, $$Y=$$ Glyoxal
B
$$X =$$ Diozonide, $$Y=$$ Succinic acid
C
$$X =$$ Monoozonide, $$Y=$$ Benzoic acid
D
$$X =$$ Triozonide, $$Y=$$ Benzaldehyde
Answer :
$$X =$$ Triozonide, $$Y=$$ Glyoxal






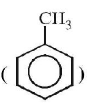 contains $$ - C{H_3}$$ group which
contains $$ - C{H_3}$$ group which