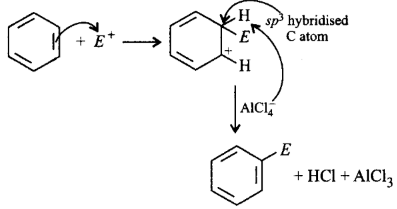
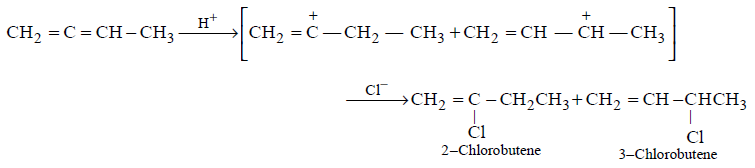71. Which of the following steps is not correct in the mechanism of electrophilic substitution of benzene?
A
Generation of electrophile like $${X^ + },{R^ + },NO_2^ + ,$$ etc.
B
Attack of electrophile resulting in the formation of arenium ion in which one of the carbon is $$s{p^3}$$ hybridised.
C
Addition of proton on benzene ring to give carbocation.
D
Removal of proton from $$s{p^3}$$ carbon atom to restore aromatic character.
Answer :
Addition of proton on benzene ring to give carbocation.
72.
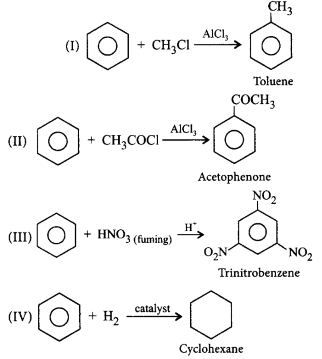
Name the products of the following reactions.
(I) $${C_6}{H_6}$$ reacts with methyl chloride in presence of $$AlC{l_3}.$$
(II) $${C_6}{H_6}$$ reacts with acetyl chloride in presence of $$AlC{l_3}.$$
(III) $${C_6}{H_6}$$ reacts with fuming nitric acid in presence of cone. $${H_2}S{O_4}.$$
(IV) $${C_6}{H_6}$$ is catalytically hydrogenated.
I
II
III
IV
(a)
Chloromethane
Toluene
Nitrobenzene
$$n$$ - Hexane
(b)
Methylbenzene
Chlorobenzene
Phenylnitrite
Trimethylbenzene
(c)
Benzyl chloride
Trimethylbenzene
Trinitrotoluene
Toluene
(d)
Toluene
Acetophenone
Trinitrobenzene
Cyclohexane
A
(a)
B
(b)
C
(c)
D
(d)
Answer :
(d)
73.
Choose the correct comparison of heat of hydrogenation for the following alkenes.
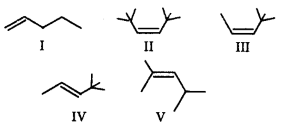
A
II < IV < III < V < I
B
III < IV < I < V < II
C
V < IV < III < I < II
D
IV < V < I < III < II
Answer :
V < IV < III < I < II
74. A mixture of 1-iodoethane and 1-iodopropane is treated with sodium metal and dry ether to carry out Wurtz reaction. Which of the following hydrocarbons will be formed?
A
Propane + Hexane
B
Ethane + Propane
C
Butane + Propane
D
Butane + Pentane + Hexane
Answer :
Butane + Pentane + Hexane
75. In the following the most stable conformation of $$n$$ - butane is
A


B


C


D


Answer :


76. Ozonolysis products of 2-pentyne after decomposition of ozonide with water and subsequent oxidation are
A
ethanoic acid and propanoic acid
B
ethanoic acid and propanone
C
ethanoic acid
D
formic acid and glyoxal
Answer :
ethanoic acid and propanoic acid
77. Which of the following will yield a mixture of 2-chlorobutene and 3-chlorobutene on treatment with $$HCl ?$$
A
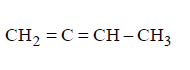
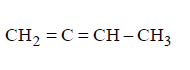
B
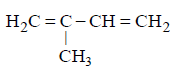
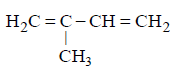
C
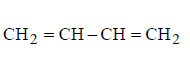
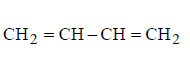
D
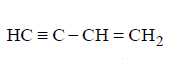
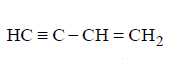
Answer :
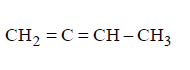
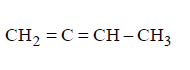
78.
The addition of $$HBr$$ to 1-butene gives a mixture of products (I), (II) and (III) :

$$\mathop {C{H_3} - C{H_2} - C{H_2} - C{H_2} - Br}\limits_{\left( {{\text{III}}} \right)} $$
The mixture consists of
A
(I) and (II) as major and (III) as minor products
B
(II) as major, (I) and (III) as minor products
C
(II) as minor, (I) and (III) as major products
D
(I) and (II) as minor and (III) as major products
Answer :
(I) and (II) as major and (III) as minor products
79. The maximum number of isomers for an alkene with the molecular formula $${C_4}{H_8}$$ is
A
2
B
3
C
4
D
5
Answer :
4
80. Chlorination of alkanes is a photochemical process. It is initiated by the process of
A
heterolysis
B
homolysis
C
pyrolysis
D
hydrolysis
Answer :
homolysis