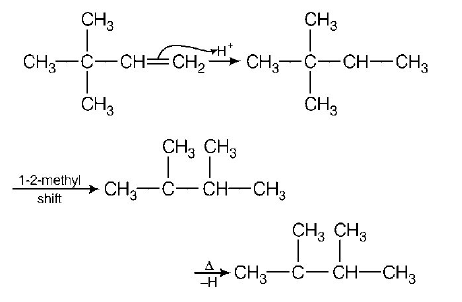
31. $$2,3-dimethyl-2-butene$$ can be prepared by heating which of the following compounds with a strong acid?
A
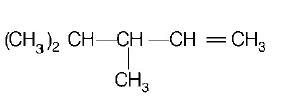

B
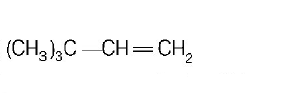
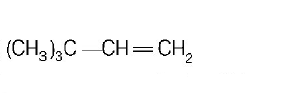
C
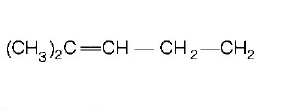
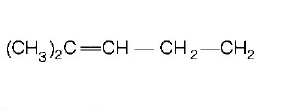
D
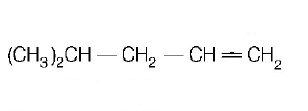

Answer :
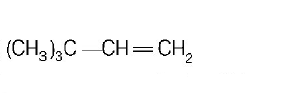
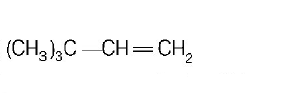
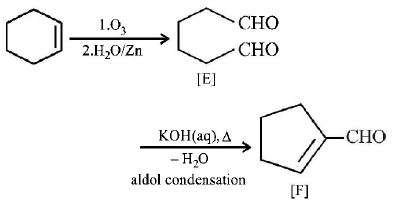
32. Cyclohexene on ozonolysis followed by reaction with zine dust and water gives compound $$E.$$ Compound $$E$$ on further treatment with aqueous $$KOH$$ yields compound $$F.$$ Compound $$F$$ is
A
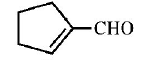

B
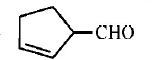

C
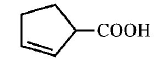

D
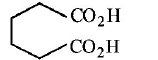
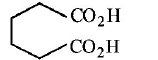
Answer :


33.
Consider the following compounds :
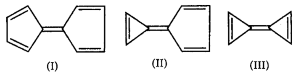
Which compound possesses highest dipole moment?
A
I
B
II
C
III
D
All have equal dipole moment
Answer :
II
34. An alkane $${C_6}{H_{14}}$$ gives two monochloro derivatives on chlorination. Its possible structure is
A
$$C{H_3}C{H_2}C{H_2}C{H_2}C{H_2}C{H_3}$$
B
\[C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
C{{H}_{3}}
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
C
\[\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
C{{H}_{2}}C{{H}_{3}}\,\,\,\,
\end{smallmatrix}}{\mathop{C{{H}_{3}}-CH-C{{H}_{2}}C{{H}_{3}}}}\,\]
D
\[C{{H}_{3}}\overset{\begin{smallmatrix}
\,\,C{{H}_{3}} \\
|\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,\overset{\begin{smallmatrix}
C{{H}_{3}}\,\, \\
|\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH-}}\,C{{H}_{3}}\]
Answer :
\[C{{H}_{3}}\overset{\begin{smallmatrix}
\,\,C{{H}_{3}} \\
|\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,\overset{\begin{smallmatrix}
C{{H}_{3}}\,\, \\
|\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH-}}\,C{{H}_{3}}\]
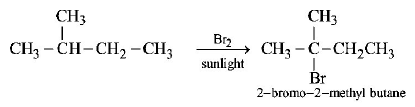
36. 2-Methylbutane on reacting with bromine in the presence of sunlight gives mainly
A
1 - bromo - 3 - methylbutane
B
2 - bromo - 3 - methylbutane
C
2 - bromo - 2 - methylbutane(d)
D
1 - bromo - 2 - methylbutane
Answer :
2 - bromo - 2 - methylbutane(d)
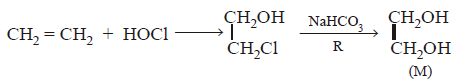
37.
In reaction sequence
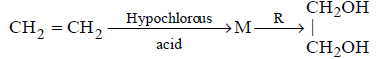
molecule $$'M'$$ and reagent $$'R'$$ respectively are
A
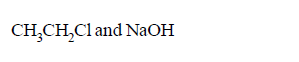
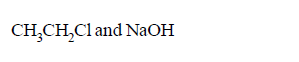
B
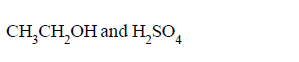
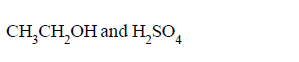
C
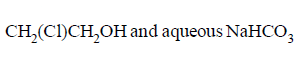
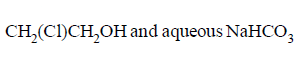
D
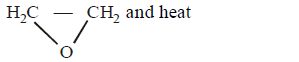
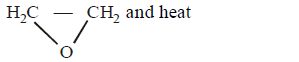
Answer :
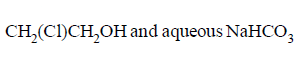
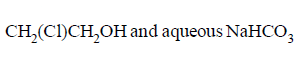
38. Butene - 1 may be converted to butane by reaction with
A
$$Sn - HCl$$
B
$$Zn - Hg$$
C
$$Pd/{H_2}$$
D
$$Zn - HCl$$
Answer :
$$Pd/{H_2}$$
39.
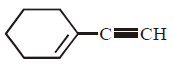
\[\xrightarrow[\left( \text{ii} \right)\,C{{H}_{3}}Br]{\left( \text{i} \right)\,NaN{{H}_{2}},\,N{{H}_{3}}}\left( A \right)\xrightarrow[\text{Lindlar catalyst}]{{{H}_{2}}}\left( B \right);\] Product $$(B)$$ is :
A


B
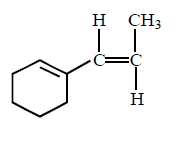

C
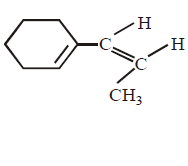

D
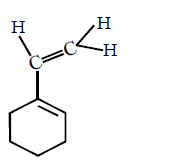

Answer :


40. \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\xrightarrow[{{170}^{\circ }}C]{\text{conc}\text{.}\,{{H}_{2}}S{{O}_{4}}}\] \[A\xrightarrow[{{500}^{\circ }}C]{C{{l}_{2}}}B.\] $$A$$ and $$B$$ are
A
$$A = C{H_3}C{H_2}C{H_3},B = C{H_3}C{H_2}C{H_2}Cl$$
B
$$A = C{H_3}CH = C{H_2},$$ $$B = C{H_2}ClCH = C{H_2}$$
C
$$A = C{H_2} = C{H_2},B = C{H_3}C{H_2}Cl$$
D
$$A = C{H_3}C{H_2}C{H_3},B = C{H_3}CH = C{H_2}$$
Answer :
$$A = C{H_3}CH = C{H_2},$$ $$B = C{H_2}ClCH = C{H_2}$$