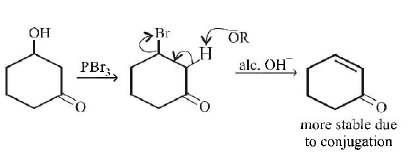
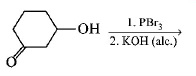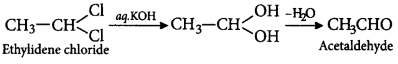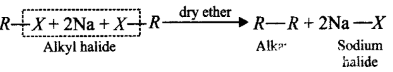71.
In the following sequence of reactions
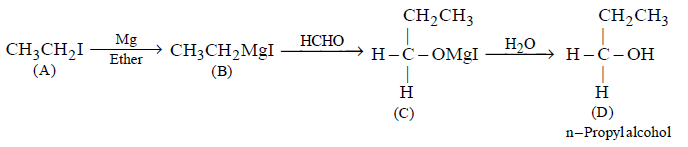
\[\underset{A}{\mathop{C{{H}_{3}}C{{H}_{2}}I}}\,\xrightarrow[\text{ether}]{Mg}B\xrightarrow{HCHO}C\xrightarrow{{{H}_{2}}O}D\]
the compound $$D$$ is
A
propanal
B
butanal
C
$$n$$ - butyl alcohol
D
$$n$$ - propyl alcohol
Answer :
$$n$$ - propyl alcohol
72.
Identify correct reactivity order for $${S_N}1$$ reaction
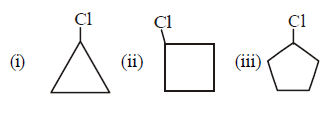
A
(i) > (ii) > (iii)
B
(ii) > (iii) > (i)
C
(i) > (iii) > (ii)
D
(iii) > (ii) > (i)
Answer :
(iii) > (ii) > (i)
74. Ethylene dichloride and ethylidene chloride are isomeric compounds. The false statement about these isomers is that they
A
are both hydrolysed to the same product
B
contain the same percentage of chlorine
C
are position isomers
D
react with alcoholic potash and give the same product
Answer :
are both hydrolysed to the same product
75. Which of the following haloalkanes is most reactive?
A
1-Chloropropane
B
1-Bromopropane
C
2-Chloropropane
D
2-Bromopropane
Answer :
2-Bromopropane
76. The reaction, \[C{{H}_{2}}=CH-C{{H}_{3}}+HBr\to \] \[C{{H}_{3}}\overset{\begin{smallmatrix} Br\,\, \\ |\,\,\,\, \end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}}\] is an example of
A
nucleophilic addition
B
free radical addition
C
electrophilic addition
D
electrophilic substitution
Answer :
electrophilic addition
77. Alkyl halides react with metallic sodium in dry ether producing
A
alkanes with same number of carbon atoms
B
alkanes with double the number of carbon atoms
C
alkenes with triple the number of carbon atoms
D
alkenes with same number of carbon atoms
Answer :
alkanes with double the number of carbon atoms
78. An alkyl chloride produces a single alkene on reaction with sodium ethoxide and ethanol. The alkene further undergoes hydrogenation to yield 2-methylbutane. Identify the alkyl chloride amongst the following compounds.
A
$$ClC{H_2}CH\left( {C{H_3}} \right)C{H_2}C{H_3}$$
B
$$ClC{H_2}C{H_2}C{H_2}C{H_3}$$
C
$$ClC{H_2}CH\left( {C{H_3}} \right)C{H_2}C{H_3}$$
D
$$C{H_3}C\left( {Cl} \right)\left( {C{H_3}} \right)C{H_2}C{H_3}$$
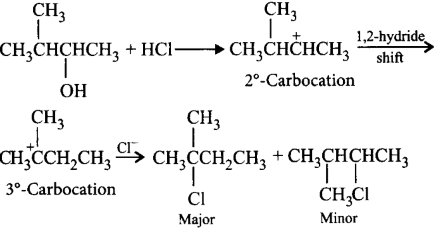
Answer :
$$ClC{H_2}CH\left( {C{H_3}} \right)C{H_2}C{H_3}$$
79.
Halogen acids react with alcohols to form alkyl halides. The reaction follows a nucleophilic substitution mechanism. What will be the major product of the following reaction?
\[C{{H}_{3}}\overset{\begin{smallmatrix}
\,\,C{{H}_{3}} \\
|\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\, \\
OH\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH-}}\,C{{H}_{3}}+HCl\to \]
A
\[\underset{\begin{smallmatrix}
| \\
\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\mathop{C{{H}_{3}}CH-}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\,\, \\
Cl\,\,\,\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH-}}\,C{{H}_{3}}\]
B
\[\underset{\begin{smallmatrix}
| \\
Cl
\end{smallmatrix}}{\mathop{C{{H}_{3}}CH-}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\, \\
C{{H}_{3}}\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH-}}\,C{{H}_{3}}\]
C
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
Cl
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,C{{H}_{2}}C{{H}_{3}}\]
D
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Cl\]
Answer :
\[C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
Cl
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,C{{H}_{2}}C{{H}_{3}}\]
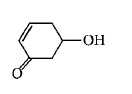
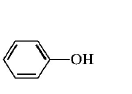
80. Which of the following is most reactive towards $${S_N}2$$ reaction ?
A
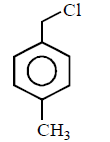

B
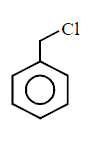

C
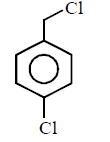

D
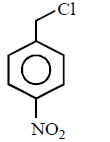

Answer :