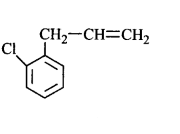
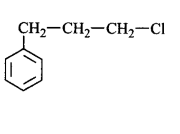
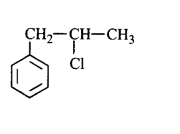
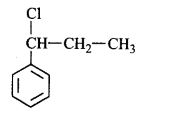
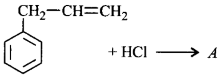51. Molecules whose mirror image is non-superimposable over them are known as chiral. Which of the following molecules is chiral in nature?
A
2-Bromobutane
B
1-Brornobutane
C
2-Bromopropane
D
2-Bromopropan-2-ol
Answer :
2-Bromobutane
53. Tertiary alkyl halides are practically inert to substitution by $${S_N}2$$ mechanism because
A
the carbocation formed is unstable
B
there is steric hindrance
C
there is inductive effect
D
the rate of reaction is faster in $${S_N}2$$ mechanism
Answer :
there is steric hindrance
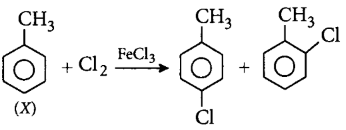
54. A compound $$X$$ with molecular formula, $${C_7}{H_8}$$ is treated with $$C{l_2}$$ in presence of $$FeC{l_3}.$$ Which of the following compounds are formed during the reaction?
A

B
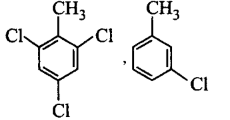

C
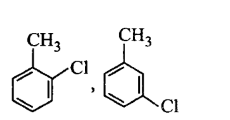

D
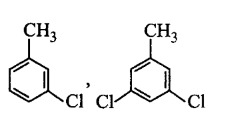

Answer :


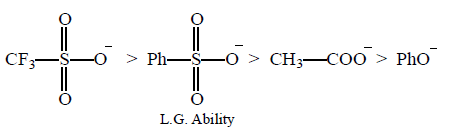
55.
Consider the following anions.
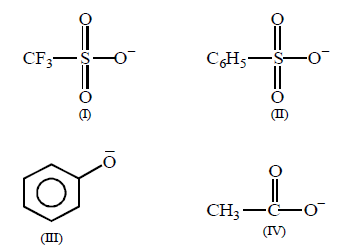
When attached to $$s{p^3}$$ -hydridized carbon, their leaving group ability in nucleophilic substitution reaction decreases in the order :
A
I > II > III > IV
B
I > II > IV > III
C
IV > I > II > III
D
IV > III > II > I
Answer :
I > II > IV > III
56. Which of the following is not an allylic halide?
A
4-Bromopent-2-ene
B
3-Bromo-2-methylbut-1-ene
C
1-Bromobut-2-ene
D
4-Bromobut-1-ene
Answer :
4-Bromobut-1-ene
57.
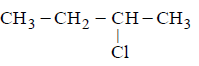 obtained by chlorination of $$n$$ - butane, will be
obtained by chlorination of $$n$$ - butane, will be
A
meso-form
B
racemic mixture
C
$$d$$ - form
D
$$l$$ - form
Answer :
racemic mixture
58.
In the reaction given below,
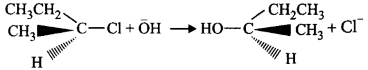
which of the following statements is correct
A
The reaction proceeds $$via\,{S_N}2$$ mechanism hence inversion of configuration takes place.
B
The reaction proceeds $$via\,{S_N}1$$ mechanism hence inversion of configuration takes place.
C
The reaction proceeds $$via\,{S_N}2$$ mechanism hence their is no change in the configuration.
D
The reaction proceeds $$via\,{S_N}1$$ mechanism hence there is no change in the configuration.
Answer :
The reaction proceeds $$via\,{S_N}2$$ mechanism hence inversion of configuration takes place.
59. In two separate experiments equal quantities of an alkyl halide, $${C_4}{H_9}Cl,$$ were treated at the same temperature with equal volume of 0.1 molar and 0.2 molar solutions of $$NaOH$$ respectively. In both the experiments, $${t_{\frac{1}{2}}}$$ of the two reactions were the same. The most likely structure of halide is
A
$$C{H_3}C{H_2}C{H_2}C{H_2}Cl$$
B
$$C{H_3}CH\left( {Cl} \right)C{H_2}C{H_3}$$
C
$${\left( {C{H_3}} \right)_2}CHC{H_2}Cl$$
D
$${\left( {C{H_3}} \right)_3}CCl$$
Answer :
$${\left( {C{H_3}} \right)_3}CCl$$
60. Which is the correct IUPAC name for \[C{{H}_{3}}\underset{\begin{smallmatrix} |\,\,\,\,\,\, \\ {{C}_{2}}{{H}_{5}} \end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}-Br?\]
A
1-Bromo-2-ethylpropane
B
1-Bromo-2-ethyl-2-methylethane
C
1-Bromo- 2-methylbutane
D
2-Methyl-1-bromobutane
Answer :
1-Bromo- 2-methylbutane