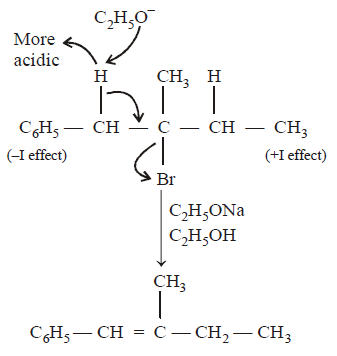
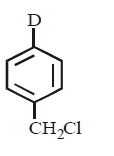
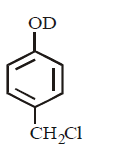
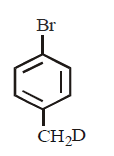
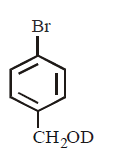
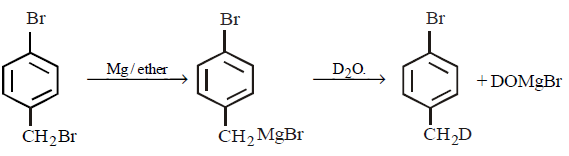
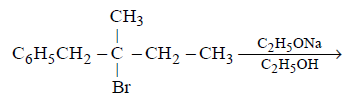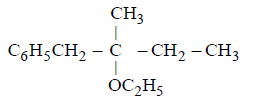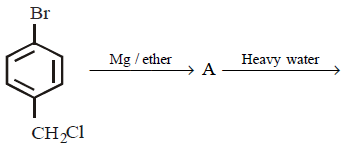21. Methyl bromide reacts with $$AgF$$ to give methyl fluoride and silver bromide. This reaction is called
A
Fittig reaction
B
Swarts reaction
C
Wurtz reaction
D
Finkelstein reaction
Answer :
Swarts reaction
23.
\[C{{H}_{3}}C{{H}_{2}}Cl\xrightarrow{NaCN}X\xrightarrow{Ni/{{H}_{2}}}Y\xrightarrow[\text{anhydride}]{\text{Acetic}}Z\]
$$Z$$ in the above reaction sequence is
A
$$C{H_3}C{H_2}C{H_2}NHCOC{H_3}$$
B
$$C{H_3}C{H_2}C{H_2}N{H_2}$$
C
$$C{H_3}C{H_2}C{H_2}CONHC{H_3}$$
D
$$C{H_3}C{H_2}C{H_2}CONHCOC{H_3}$$
Answer :
$$C{H_3}C{H_2}C{H_2}NHCOC{H_3}$$
25. Bottles containing $${C_6}{H_5}I$$ and $${C_6}{H_5}C{H_2}I$$ lost their original labels. They were labelled $$A$$ and $$B$$ for testing. $$A$$ and $$B$$ were separately taken in test tubes and boiled with $$NaOH$$ solution. The end solution in each tube was made acidic with dilute $$HN{O_3}$$ and some $$AgN{O_3}$$ solution added. Solution $$B$$ gave a yellow precipitate. Which one of the following statements is true for the experiment?
A
Addition of $$HN{O_3}$$ was unnecessary
B
$$A$$ was $${C_6}{H_5}I$$
C
$$A$$ was $${C_6}{H_5}C{H_2}I$$
D
$$A$$ and $$B$$ can't be predicted by this experiment
Answer :
$$A$$ was $${C_6}{H_5}I$$
26. 2-Chloro-2-methylpropane on reaction with alc. $$KOH$$ gives $$X$$ as the product. $$X$$ is
A
but-2-ene
B
2-methylbut -1-ene
C
2-methylprop-1-ene
D
2-methylbutan-2-ol
Answer :
2-methylprop-1-ene
27. Aryl fluoride may be prepared from arene diazonium chloride using :
A
$$HB{F_4}/\Delta $$
B
$$HB{F_4}/NaN{O_2},Cu,\Delta $$
C
$$CuF/HF$$
D
$$Cu/HF$$
Answer :
$$HB{F_4}/\Delta $$
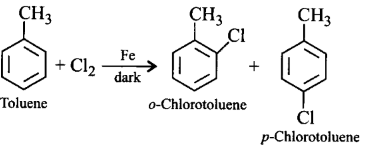
28. The reaction of toluene with chlorine in the presence of iron and in the absence of light yields __________.
A

B


C


D


Answer :


29. Which of the following products does not match correctly with the reaction?
A


B


C


D


Answer :


30. The reaction conditions leading to the best yields of $${C_2}{H_5}Cl$$ are :
A
\[{{C}_{2}}{{H}_{6}}\text{(excess)}+C{{l}_{2}}\xrightarrow{UV\,\,\text{light}}\]
B
\[{{C}_{2}}{{H}_{6}}+C{{l}_{2}}\xrightarrow[\text{room}\,\,\text{temperature}]{\text{dark}}\]
C
\[{{C}_{2}}{{H}_{6}}+C{{l}_{2}}\text{(excess)}\xrightarrow{UV\,\,\text{light}}\]
D
\[{{C}_{2}}{{H}_{6}}+C{{l}_{2}}\xrightarrow{UV\,\,\text{light}}\]
Answer :
\[{{C}_{2}}{{H}_{6}}\text{(excess)}+C{{l}_{2}}\xrightarrow{UV\,\,\text{light}}\]