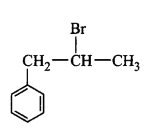
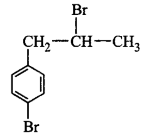
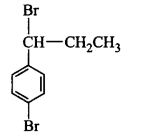
11. The IUPAC name of $${\left( {C{H_3}} \right)_2}CH - C{H_2} - C{H_2}Br$$ is
A
1-bromopentane
B
1-bromo- 3-methylbutane
C
2-methyl-4-bromobutane
D
2-methyl-3-bromopropane
Answer :
1-bromo- 3-methylbutane
12. Cyanide ion acts as an ambident nucleophile. From which end it acts as a stronger nucleophile in aqueous medium?
A
It acts as a stronger nucleophile from carbon end.
B
It acts as a stronger nucleophile from nitrogen end.
C
It depends on the nature of the alkyl halide.
D
It has same strength from both the ends.
Answer :
It acts as a stronger nucleophile from carbon end.
13. \[{{C}_{2}}{{H}_{5}}Br\xrightarrow{AgCN}X\xrightarrow[Zn-Hg/HCl]{\text{Reduction}}Y,\] Here $$Y$$ is
A
Ethyl methyl amine
B
$$n$$ - propylamine
C
Isopropylamine
D
Ethylamine
Answer :
Ethyl methyl amine
14. Which of the following alkyl halides undergoes hydrolysis with aqueous $$KOH$$ at the fastest rate?
A
$$C{H_3}C{H_2}C{H_2}Cl$$
B
$$C{H_3}C{H_2}Cl$$
C
$$C{H_3}C{H_2}C{H_2}C{H_2}Cl$$
D
$$C{H_3}C{H_2}CH\left( {Br} \right)C{H_3}$$
Answer :
$$C{H_3}C{H_2}CH\left( {Br} \right)C{H_3}$$
15. The order of reactivities of methyl halides in the formation of Grignard reagent is
A
$$C{H_3}I > C{H_3}Br > C{H_3}Cl$$
B
$$C{H_3}Cl > C{H_3}Br > C{H_3}I$$
C
$$C{H_3}Br > C{H_3}Cl > C{H_3}I$$
D
$$C{H_3}Br > C{H_3}I > C{H_3}Cl$$
Answer :
$$C{H_3}I > C{H_3}Br > C{H_3}Cl$$
16. An unknown alkyl halide $$(A)$$ reacts with alcoholic $$KOH$$ to produce a hydrocarbon $$\left( {{C_4}{H_8}} \right)$$ as the major product. Ozonolysis of the hydrocarbon affords one mole of propanaldehyde and one mole of formaldehyde. Suggest which organic compound among the following is the correct structure of the above alkyl halide $$(A)?$$
A
$$C{H_3}CHBrC{H_2}C{H_3}$$
B
$$C{H_3}CH\left( {Br} \right)CH\left( {Br} \right)C{H_3}$$
C
$$C{H_3}C{H_2}C{H_2}C{H_2}Br$$
D
$$Br{\left( {C{H_2}} \right)_4}Br$$
Answer :
$$C{H_3}C{H_2}C{H_2}C{H_2}Br$$
18.
Which of the following is a key intermediate in the reaction shown below ?

A
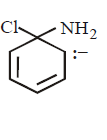

B
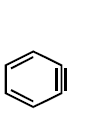

C
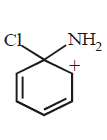

D
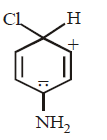

Answer :


19.
Consider the following $${S_N}2$$ reactions
$$\eqalign{
& \left( {\text{i}} \right)RX + {Y^ - } \to R - Y + {X^ - } \cr
& \left( {{\text{ii}}} \right)RX + Y \to R - {Y^ + } + {X^ - } \cr
& \left( {{\text{iii}}} \right)R{X^ + } + {Y^ - } \to R - Y + X \cr
& \left( {{\text{iv}}} \right)R{X^ + } + Y \to R - {Y^ + } + X \cr} $$
In which reactions there is large increase and large decrease in rate of reaction respectively with increase in polarity of the solvent?
A
(ii) and (iii)
B
(ii) and (iv)
C
(i) and (iv)
D
(iv) and (i)
Answer :
(ii) and (iii)
20. In $${S_N}2$$ reactions the sequence of bond breaking and bond formation is as follows
A
bond breaking is followed by formation
B
bond formation is followed by breaking
C
bond breaking and formation occur simultaneously
D
bond breaking and formation take place randomly
Answer :
bond breaking and formation occur simultaneously
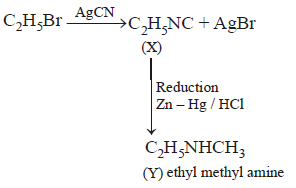
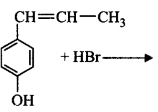 $$X, X$$ in the reaction is
$$X, X$$ in the reaction is