251. An element of group 14 forms two oxides one of which is highly poisonous and neutral. Other oxide can be easily liquefied and compressed to give a solid which is used as a refrigerant under the name of drikold. The element and the oxides are
A
$$Si,SiO,Si{O_2}$$
B
$$Pb,PbO,Pb{O_2}$$
C
$$C,CO,C{O_2}$$
D
$$Sn,SnO,Sn{O_2}$$
Answer :
$$C,CO,C{O_2}$$
252. Catenation i.e., linking of similar atoms depends on size and electronic configuration of atoms. The tendency of catenation in Group 14 elements follows the order
A
$$C > Si > Ge > Sn$$
B
$$C > > Si > Ge \approx Sn$$
C
$$Si > C > Sn > Ge$$
D
$$Ge> Sn > Si > C$$
Answer :
$$C > > Si > Ge \approx Sn$$
253.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
$$Cl{F_3}$$
1.
Pentagonal bipyramidal
b.
$$I{F_5}$$
2.
Square pyramidal
c.
$$I{F_7}$$
3.
Bent T-shaped
d.
$$BrF$$
4.
Linear
A
a - 3, b - 1, c - 4, d - 2
B
a - 1, b - 2, c - 3, d - 4
C
a - 2, b - 4, c - 3, d - 1
D
a - 3, b - 2, c - 1, d - 4
Answer :
a - 3, b - 2, c - 1, d - 4
254. Boric acid is an acid because its molecule
A
contains replaceable $${H^ + }$$ ion
B
gives up a proton
C
accepts $$O{H^ - }$$ from water releasing proton
D
combines with proton from water molecule
Answer :
accepts $$O{H^ - }$$ from water releasing proton
255. Which of the following statements regarding sulphur is incorrect?
A
$${S_2}$$ molecule is paramagnetic.
B
The vapour at $${200^ \circ }C$$ consists mostly of $${{\text{S}}_8}$$ rings.
C
At $${600^ \circ }C$$ the gas mainly consists of $${S_2}$$ molecules.
D
The oxidation state of sulphur is never less than $$ + 4$$ in
its compounds.
Answer :
The oxidation state of sulphur is never less than $$ + 4$$ in
its compounds.
256. $$FeC{l_3}$$ solution on reaction with $$S{O_2}$$ changes to
A
$$FeC{l_2}$$
B
$$F{e_2}{\left( {S{O_4}} \right)_3}$$
C
$$F{e_2}{\left( {S{O_3}} \right)_3}$$
D
$$FeS{O_4}$$
Answer :
$$FeC{l_2}$$
257. $$KF$$ combines with $$HF$$ to form $$KH{F_2},$$ The compound contains the species.
A
$${K^ + },{F^ - }{\text{and}}\,{H^ + }$$
B
$${K^ + },{F^ - }{\text{and}}\,HF$$
C
$${K^ + }\,{\text{and }}{\left[ {H{F_2}} \right]^ - }$$
D
$${\left[ {KHF} \right]^ + }\,{\text{and}}\,{F^ - }$$
Answer :
$${K^ + }\,{\text{and }}{\left[ {H{F_2}} \right]^ - }$$
258.
In $$B{X_3},B - X$$ distance is shorter than what is expected theoretically because $$\left( {X = F,Cl , Br,I} \right)$$
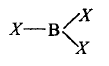
A
$$s{p^3}$$ hybridisation of $$B$$ is responsible for shorter $$B - X$$ distance.
B
$$B - X$$ has a double bond character due to back - bonding.
C
Dimerisation takes place in $$B{X_3}$$ which is responsible for shorter $$B - X$$ distance.
D
Due to large size of $$X, B - X$$ distance decreases
Answer :
$$B - X$$ has a double bond character due to back - bonding.
259. Which one of the following arrangements represents the correct order of electron gain enthalpy ( with negative sign ) of the given atomic species ?
A
$$Cl < F < S < O$$
B
$$O < S < F < Cl$$
C
$$S < O < Cl < F$$
D
$$F < Cl < O < S$$
Answer :
$$O < S < F < Cl$$
260.
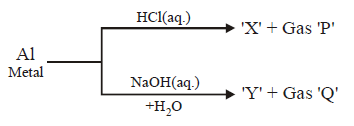
The incorrect statement regarding above reactions is :
A
$$Al$$ shows amphoteric character
B
Gas $$'P'$$ and $$'Q'$$ are different
C
Both $$X$$ and $$Y$$ are water soluble
D
Gas $$Q$$ is inflammable
Answer :
Gas $$'P'$$ and $$'Q'$$ are different