281. Stability of the species $$L{i_2},Li_2^ - $$ and $$Li_2^ + $$ increases in the order of:
A
$$L{i_2} < Li_2^ + < Li_2^ - $$
B
$$Li_2^ - < Li_2^ + < L{i_2}$$
C
$$L{i_2} < Li_2^ - < Li_2^ + $$
D
$$Li_2^ - < L{i_2} < Li_2^ + $$
Answer :
$$Li_2^ - < Li_2^ + < L{i_2}$$
282. Which of the following molecules has the maximum dipole moment?
A
$$C{O_2}$$
B
$$C{H_4}$$
C
$$N{H_3}$$
D
$$N{F_3}$$
Answer :
$$N{F_3}$$
283. Which of the following compounds does not the violate octet rule?
A
$$Br{F_5}$$
B
$$S{F_6}$$
C
$$I{F_7}$$
D
$$PCl_4^ + $$
Answer :
$$PCl_4^ + $$
284. In $$PO_4^{3 - }$$ ion, the formal charge on the oxygen atom of $$P-O$$ bond is
A
+ 1
B
- 1
C
- 0.75
D
+ 0.75
Answer :
- 0.75
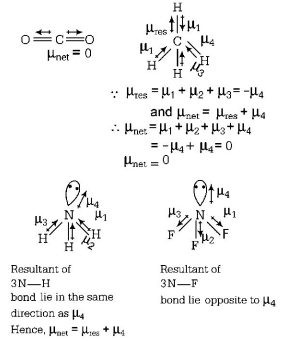
285. Though covalent in nature, methanol is soluble in water, why?
A
Methanol is transparent like water.
B
Due to hydrogen bonding between methanol and water molecules.
C
Due to van der Waals' forces between methanol and water.
D
Due to covalent attraction forces.
Answer :
Due to hydrogen bonding between methanol and water molecules.
286. Which of the following molecular orbitals has two nodal planes ?
A
$$\sigma 2s$$
B
$$\pi 2{p_y}$$
C
$${\pi ^ * }2{p_y}$$
D
$${\sigma ^ * }2{p_x}$$
Answer :
$${\pi ^ * }2{p_y}$$
287. Which type of hybridisation is shown by carbon atoms from left to right in the given compound $$C{H_2} = CH - C \equiv N?$$
A
$$s{p^2},s{p^2},sp$$
B
$$s{p^2},sp,sp$$
C
$$sp,s{p^2},s{p^3}$$
D
$$s{p^3},s{p^2},sp$$
Answer :
$$s{p^2},s{p^2},sp$$
288. Which of the following is not a correct statement?
A
The electron deficient molecules can act as Lewis acids
B
The canonical structures have no real existence
C
Every $$A{B_5}$$ molecule does infact have square pyramid structure
D
Multiple bonds are always shorter than corresponding single bond
Answer :
Every $$A{B_5}$$ molecule does infact have square pyramid structure
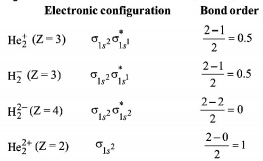
289. According to molecular orbital theory, which of the following will not be a viable molecule?
A
\[He_2^{2 + }\]
B
\[He_2^ + \]
C
\[H_2^ - \]
D
\[H_2^{2 - }\]
Answer :
\[H_2^{2 - }\]
290. Identify the least stable ion amongst the following :
A
$$L{i^ - }$$
B
$$B{e^ - }$$
C
$${B^ - }$$
D
$${C^ - }$$
Answer :
$$B{e^ - }$$
 $$ = 8\,{\text{electrons}}$$
$$ = 8\,{\text{electrons}}$$
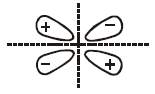 It has two nodal planes. It is $${\pi ^ * }2{p_y}$$
It has two nodal planes. It is $${\pi ^ * }2{p_y}$$