211. Atomic orbitals of carbon in carbon dioxide are
A
$$s{p^2}$$ - hybridised
B
$$s{p^3}d$$ - hybridised
C
$$sp$$ - hybridised
D
$$s{p^3}$$ - hybridised
Answer :
$$sp$$ - hybridised
212. Which of the following statements is true about hybridisation?
A
The hybridised orbitals have different energies for each orbital.
B
The number of hybrid orbitals is equal to the number of atomic orbitals that are hybridised.
C
Hybrid orbitals form multiple bonds.
D
The orbitals with different energies undergo hybridisation.
Answer :
The number of hybrid orbitals is equal to the number of atomic orbitals that are hybridised.
213. The hydrogen bond is strongest in :
A
$$O - H......S$$
B
$$S - H.......O$$
C
$$F - H......F$$
D
$$F - H.......O$$
Answer :
$$F - H......F$$
214.
What is the formal charge on carbon atom in the following two structures :

A
0, - 2
B
0, 0
C
+ 2, -2
D
+ 1, - 1
Answer :
0, 0
215. Among the following chloro-compound having the lowest dipole moment is
A


B


C


D


Answer :


216. Which of the following statements is not correct?
A
Double bond is shorter than a single bond
B
Sigma bond is weaker than a $$\pi $$ -bond
C
Double bond is stronger than a single bond
D
Covalent bond is stronger than hydrogen bond
Answer :
Sigma bond is weaker than a $$\pi $$ -bond
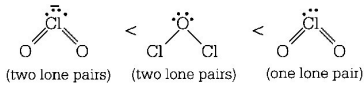
217. The correct order of increasing bond angles in the following species is
A
$$C{l_2}O < Cl{O_2} < ClO_2^ - $$
B
$$Cl{O_2} < C{l_2}O < ClO_2^ - $$
C
$$C{l_2}O < ClO_2^ - < Cl{O_2}$$
D
$$ClO_2^ - < C{l_2}O < Cl{O_2}$$
Answer :
$$ClO_2^ - < C{l_2}O < Cl{O_2}$$
218. Among the following ions the $$p\pi - d\pi $$ overlap could be present in
A
$$NO_2^ - $$
B
$$NO_3^ - $$
C
$$PO_4^{3 - }$$
D
$$CO_3^{2 - }$$
Answer :
$$PO_4^{3 - }$$
219. Among $$LiCl,BeC{l_2},BC{l_3}$$ and $$CC{l_4},$$ the covalent bond character follows the order
A
$$LiCl < BeC{l_2} > BC{l_3} > \,CC{l_4}$$
B
$$LiCl < BeC{l_2} < BC{l_3} > \,CC{l_4}$$
C
$$LiCl < BeC{l_2} < BC{l_3} < \,CC{l_4}$$
D
$$LiCl > BeC{l_2} > BC{l_3} > \,CC{l_4}$$
Answer :
$$LiCl < BeC{l_2} < BC{l_3} < \,CC{l_4}$$
220. Which of the following statements is not true?
A
Intermolecular hydrogen bonds are formed between two different molecules of compounds.
B
Intramolecular hydrogen bonds are formed between two different molecules of the same compound.
C
Intramolecular hydrogen bonds are formed within the same molecule.
D
Hydrogen bonds have strong influence on the physical properties of a compound.
Answer :
Intramolecular hydrogen bonds are formed between two different molecules of the same compound.