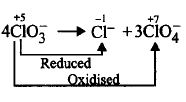
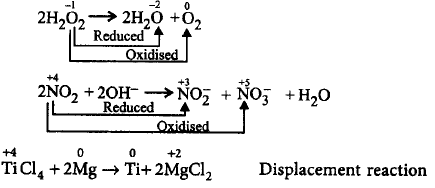
11. Which of the following is not an example of disproportionation reaction?
A
$$4ClO_3^ - \to C{l^ - } + 3ClO_4^ - $$
B
$$2{H_2}{O_2} \to 2{H_2}O + {O_2}$$
C
$$2N{O_2} + 2O{H^ - } \to $$ $$NO_2^ - + NO_3^ - + {H_2}O$$
D
$$TiC{l_4} + 2Mg \to Ti + 2MgC{l_2}$$
Answer :
$$TiC{l_4} + 2Mg \to Ti + 2MgC{l_2}$$
12.
In the reaction : $$C + 4HN{O_3} \to C{O_2} + 2{H_2}O + 4N{O_2}$$
$$HN{O_3}$$ act as
A
an oxidizing agent
B
an acid
C
an acid as well as oxidizing agent
D
a reducing agent.
Answer :
an oxidizing agent
13. Among $$N{H_3},HN{O_3},Na{N_3}$$ and $$M{g_3}{N_2}$$ the number of molecules having nitrogen in negative oxidation state is
A
1
B
2
C
3
D
4
Answer :
3
14. Oxidation state of sulphur in anions $${S_2}O_3^{2 - },{S_2}O_4^{2 - }$$ and $${S_2}O_6^{2 - }$$ increases in the orders :
A
$${S_2}O_6^{2 - } < {S_2}O_4^{2 - } < SO_3^{2 - }$$
B
$$SO_6^{2 - } < {S_2}O_4^{2 - } < {S_2}O_6^{2 - }$$
C
$${S_2}O_4^{2 - } < SO_3^{2 - } < {S_2}O_6^{2 - }$$
D
$${S_2}O_4^{2 - } < {S_2}O_6^{2 - } < SO_3^{2 - }$$
Answer :
$${S_2}O_4^{2 - } < SO_3^{2 - } < {S_2}O_6^{2 - }$$
15. The standard electrode potentials of four elements $$A, B, C$$ and $$D$$ are –3.05, –1.66, –0.40 and +0.80. The highest chemical reactivity will be exhibited by :
A
$$A$$
B
$$B$$
C
$$C$$
D
$$D$$
Answer :
$$A$$
16.
$$a{K_2}C{r_2}{O_7} + bKCl + c{H_2}S{O_4} \to $$ $$xCr{O_2}C{l_2} + yKHS{O_4} + z{H_2}O$$
The above equation balances when
A
$$a = 2,b = 4,c = 6\,\,{\text{and}}\,x = 2,y = 6,z = 3$$
B
$$a = 4,b = 2,c = 6\,{\text{and}}\,x = 6,y = 2,z = 3$$
C
$$a = 6,b = 4,c = 2\,{\text{and}}\,x = 6,y = 3,z = 2$$
D
$$a = 1,b = 4,c = 6\,{\text{and}}\,x = 2,y = 6,z = 3$$
Answer :
$$a = 1,b = 4,c = 6\,{\text{and}}\,x = 2,y = 6,z = 3$$
17.
Identify the compounds which are reduced and oxidised in the following reaction :
$$3{N_2}{H_4} + 2BrO_3^ - \to $$ $$3{N_2} + 2B{r^ - } + 6{H_2}O$$
A
$${N_2}{H_4}$$ is oxidised and $$BrO_3^ - $$ is reduced.
B
$$BrO_3^ - $$ is oxidised and $${N_2}{H_4}$$ is reduced.
C
$$BrO_3^ - $$ is both reduced and oxidised.
D
$${N_2}{H_4}$$ is both reduced and oxidised.
Answer :
$${N_2}{H_4}$$ is oxidised and $$BrO_3^ - $$ is reduced.
18. Which of the following is not a rule for calculating oxidation number?
A
For ions, oxidation number is equal to the charge on the ion.
B
The oxidation number of oxygen is -2 in all of its compounds.
C
The oxidation number of fluorine is -1 in all of its compounds.
D
Oxidation number of hydrogen is +1 except in binary hydrides of alkali metals and alkaline earth metals where it is -1.
Answer :
The oxidation number of oxygen is -2 in all of its compounds.
19. Using the standard electrode potential, find out the pair between which redox reaction is not feasible. $${E^ \circ }$$ values : $$\frac{{F{e^{3 + }}}}{{F{e^{2 + }}}} = + 0.77;\frac{{{I_2}}}{{{I^ - }}} = + 0.54;$$ $$\frac{{C{u^{2 + }}}}{{Cu}} = + 0.34;\frac{{A{g^ + }}}{{Ag}} = + 0.80\,V$$
A
$$F{e^{3 + }}\,{\text{and}}\,\,{I^ - }$$
B
$$A{g^ + }\,{\text{and}}\,\,Cu$$
C
$$F{e^{3 + }}\,{\text{and}}\,\,Cu$$
D
$$Ag\,{\text{and}}\,\,F{e^{3 + }}$$
Answer :
$$Ag\,{\text{and}}\,\,F{e^{3 + }}$$
20. In the reaction : $$C{l_2} + O{H^ - } \to C{l^ - } + ClO_4^ - + {H_2}O$$
A
chlorine is oxidised
B
chlorine is reduced
C
chlorine is oxidised as well as reduced
D
chlorine is neither oxidised nor reduced
Answer :
chlorine is oxidised as well as reduced