191. Electronegativity of carbon atoms depends upon their state of hybridisation. In which of the following compounds, the carbon marked with asterisk is most electronegative?
A
$$C{H_3} - C{H_2} - \mathop C\limits^ * {H_2} - C{H_3}$$
B
$$C{H_3} - \mathop C\limits^ * H = CH - C{H_3}$$
C
$$C{H_3} - C{H_2} - C \equiv \mathop C\limits^ * H$$
D
$$C{H_3} - C{H_2} - CH = \mathop C\limits^ * {H_2}$$
Answer :
$$C{H_3} - C{H_2} - C \equiv \mathop C\limits^ * H$$
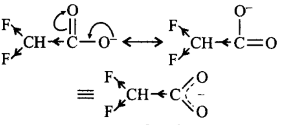
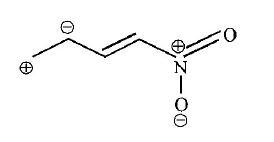
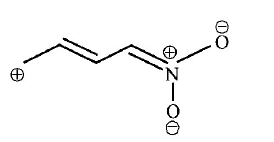
192. Ionic species are stabilised by the dispersal of charge. Which of the following carboxylate ion is the most stable?
A
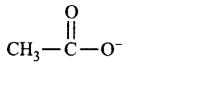
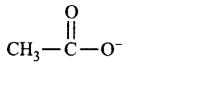
B
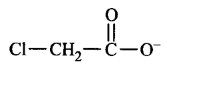
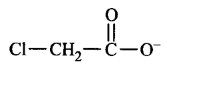
C


D


Answer :


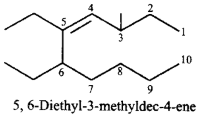
193.
The correct IUPAC name of the compound 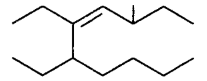 is
is
A
3-heptyl-5-methylhept-3-ene
B
5, 6-diethyl-3-methyldec-4-ene
C
5-butyl-3-methyloct-4-ene
D
8-methyl-3-propylhex-3-ene
Answer :
5, 6-diethyl-3-methyldec-4-ene
194. Which one of the following compounds has non zero dipole moment ?
A
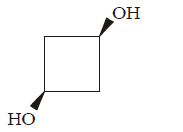

B
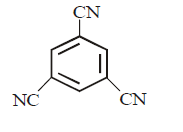

C


D


Answer :


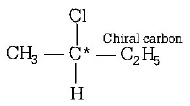
195. Which of the following is an optically active compound?
A
1 - butanol
B
1 - propanol
C
2 - chlorobutane
D
4 - hydroxybutanal
Answer :
2 - chlorobutane
196. Among the following, the compound that can be most readily sulphonated is
A
benzene
B
nitrobenzene
C
toluene
D
chlorobenzene
Answer :
toluene
198. $$0.92\,g$$ of an organic compound was analysed by combustion method. The mass of the $$U$$ - tube increased by $$1.08\,g.$$ What is the percentage of hydrogen in the compound?
A
$$13.04\% $$
B
$$52.17\% $$
C
$$65.21\% $$
D
$$11.30\% $$
Answer :
$$13.04\% $$
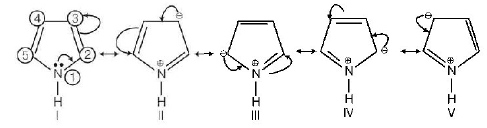
199.
In pyrrole
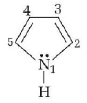
the electron density is maximum on
A
2 and 3
B
3 and 4
C
2 and 4
D
2 and 5
Answer :
2 and 5
200. The bond between carbon atom (1) and carbon atom (2) in compound $$N = \mathop C\limits_1 - \mathop {CH}\limits_2 = C{H_2}$$ involves the hybrids as
A
$$s{p^2}\,{\text{and}}\,s{p^2}$$
B
$$s{p^{3\,}}{\text{and}}\,sp$$
C
$$sp\,{\text{and}}\,s{p^2}$$
D
$$sp\,{\text{and}}\,sp$$
Answer :
$$sp\,{\text{and}}\,s{p^2}$$