231. An $${S_N}2$$ reaction at an asymmetric carbon of a compound always gives
A
an enantiomer of the substrate
B
a product with opposite optical rotation
C
a mixture of diastereomers
D
a single stereoisomer
Answer :
a single stereoisomer
232.
In the following reaction,

the structure of the major product $$'X’$$ is
A


B


C


D


Answer :


233. Which one of the following can exhibit $$cis-trans-isomerism?$$
A
$$C{H_3} - CHCl - COOH$$
B
$$H - C \equiv C - Cl$$
C
$$Cl - CH = CHCl$$
D
$$ClC{H_2} - C{H_2}Cl$$
Answer :
$$Cl - CH = CHCl$$
234. Which of the following is the most correct electron displacement for a nucleophilic reaction to take Place?
A


B


C


D


Answer :


235. Which one of the following is most reactive towards electrophilic attack?
A


B


C


D


Answer :


236.
Pick up the correct statement regarding the following resonating structures of the anilinium ion

A
Structure II is not acceptable because carbonium ions are less stable than ammonium ions
B
II is not acceptable because it is nonaromatic
C
II is not acceptable because here nitrogen has 10 valence electrons
D
II is an acceptable canonical structure.
Answer :
II is not acceptable because here nitrogen has 10 valence electrons
237. Which of the following statements is not true about the stability of carbanions?
A
Stability of carbanions increases with increase in $$s$$ - character of orbital.
B
The electron withdrawing groups like $$ - N{O_2},-CN,$$ 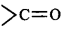 increases the stability of carbanions.
increases the stability of carbanions.
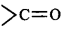 increases the stability of carbanions.
increases the stability of carbanions. C
Order of stability of carbanions is $${3^ \circ } > {2^ \circ } > {1^ \circ }.$$
D
The negatively charged carbon is $$s{p^3}$$ hybridised and pyramidal.
Answer :
Order of stability of carbanions is $${3^ \circ } > {2^ \circ } > {1^ \circ }.$$
239.
The IUPAC name of the compound shown below is 
A
2-bromo-6-chlorocyclohex-1-ene
B
6-bromo-2-chlorocyclohexene
C
3-bromo-1-chlorocyclohexene
D
1-bromo-3-chlorocyclohexene
Answer :
3-bromo-1-chlorocyclohexene
240. The IUPAC name of the compound having the formula $$CH \equiv C - CH = C{H_2}$$ is
A
$$3 - butene - 1 - yne$$
B
$$1 - butyn - 3 - ene$$
C
$$but - 1 - yne - 3 - ene$$
D
$$1 - butene - 3 - yne$$
Answer :
$$1 - butene - 3 - yne$$





