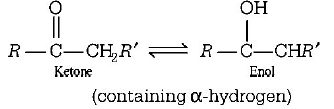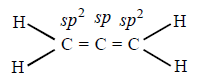181. The correct statement regarding a carbonyl compound with a hydrogen atom on its alpha-carbon, is
A
a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as aldehyde-ketone equilibration
B
a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as carbonylation
C
a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as keto-enol tautomerism
D
a carbonyl compound with a hydrogen atom on its alpha-carbon never equilibrates with its corresponding enol
Answer :
a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as keto-enol tautomerism
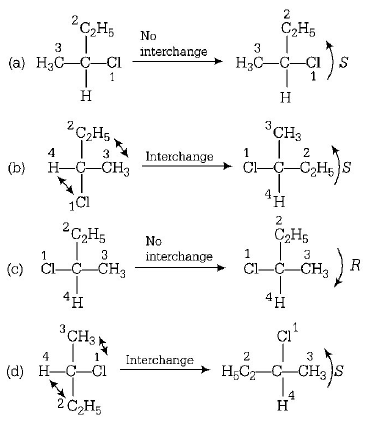
182. $$C{H_3} - CHCl - C{H_2} - C{H_3}$$ has a chiral centre. Which one of the following represents its $$R$$ -configuration?
A
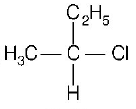

B
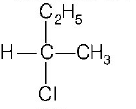

C
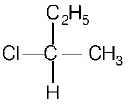

D
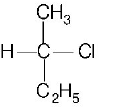

Answer :


183. Which of the following has the most acidic hydrogen ?
A
3 - Hexanone
B
2, 4 - Hexanedione
C
2, 5 - Hexanedione
D
2, 3 - Hexanedione
Answer :
2, 4 - Hexanedione
184. Isomers of a substance must have the same
A
structural formula
B
physical properties
C
chemical properties
D
molecular formula
Answer :
molecular formula
185. In allene $$\left( {{C_3}{H_4}} \right),$$ the type$$(s)$$ of hybridization of the carbon atoms is (are) :
A
$$sp\,{\text{and}}\,s{p^3}$$
B
$$s{p^2}\,{\text{and}}\,sp$$
C
$${\text{only}}\,s{p^2}$$
D
$$s{p^2}\,{\text{and}}\,s{p^3}$$
Answer :
$$s{p^2}\,{\text{and}}\,sp$$
186.
What are the hybridization and shapes of the following molecules?
$$\eqalign{
& \left( {\text{i}} \right)C{H_3}F \cr
& \left( {{\text{ii}}} \right)HC \equiv N \cr} $$
A
$$\left( {\text{i}} \right)s{p^2},$$ trigonal planar; $$\left( {{\text{ii}}} \right)s{p^3},$$ tetrahedral
B
$$\left( {\text{i}} \right)s{p^3},$$ tetrahedral ; $$\left( {{\text{ii}}} \right)sp,$$ linear
C
$$\left( {\text{i}} \right)sp,$$ linear ; $$\left( {{\text{ii}}} \right)s{p^2},$$ trigonal planar
D
$$\left( {\text{i}} \right)s{p^2},$$ trigonal planar, $$\left( {{\text{ii}}} \right)s{p^2},$$ trigonal planar
Answer :
$$\left( {\text{i}} \right)s{p^3},$$ tetrahedral ; $$\left( {{\text{ii}}} \right)sp,$$ linear
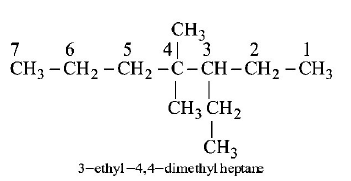
187.
The IUPAC name of 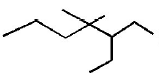 is
is
A
3 - ethyl - 4 - 4 - dimethylheptane
B
1, 1- diethyl - 2, 2 - dimethylpentane
C
4, 4 - dimethyl - 5, 5 - diethylpentane
D
5, 5 - diethyl - 4, 4 - dimethylpentane.
Answer :
3 - ethyl - 4 - 4 - dimethylheptane
188. The number of sigma and pi-bonds in 1-butene-3-yne are :
A
5 sigma and 5 pi
B
7 sigma and 3 pi
C
8 sigma and 2 pi
D
5 sigma and 4 pi
Answer :
7 sigma and 3 pi
189. Correct the increasing order of acidity is as
A
$${H_2}O,{C_2}{H_2},{H_2}C{O_3},phenol$$
B
$${C_2}{H_2},{H_2}O,{H_2}C{O_3},phenol$$
C
$$phenol,{C_2}{H_2},{H_2}C{O_3},{H_2}O$$
D
$${C_2}{H_2},{H_2}O,phenol\,{\text{and}}\,{H_2}C{O_3}$$
Answer :
$${C_2}{H_2},{H_2}O,phenol\,{\text{and}}\,{H_2}C{O_3}$$
190. Separation of two substances by crystallisation depends upon their differences in
A
densities
B
solubility
C
melting points
D
boiling points
Answer :
solubility