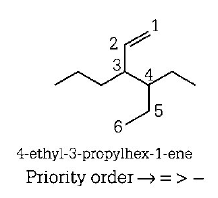
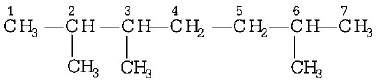171.
The correct IUPAC name of the compound
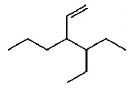 is
is
A
$$3 - ethyl - 4 - ethenylhep\tan e$$
B
$$3 - ethyl - 4 - propylhex - 5 - ene$$
C
$$3 - (\,1 - ethyl{\text{ }}propyl\,){\text{ }}hex - 1 - ene$$
D
$$4 - ethyl - 3 - propylhex - 1 - ene$$
Answer :
$$4 - ethyl - 3 - propylhex - 1 - ene$$
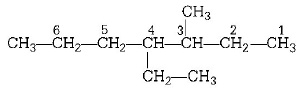
172.
The IUPAC name of
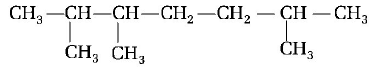
is
A
1, 3-isopropyl-3-methyl propane
B
2, 3, 6-trimethyl heptane
C
2, 5, 6-trimethyl heptane
D
2, 6, 3-trimethyl heptane
Answer :
2, 3, 6-trimethyl heptane
173. Names of some compounds are given. Which one is not correct in IUPAC system?
A


B
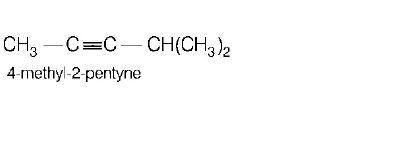
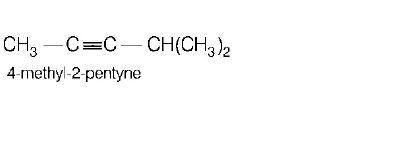
C
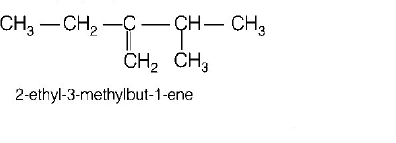

D
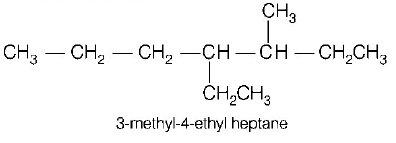

Answer :


174. Stability of alkyl carbocations can be explained by
A
inductive effect only
B
hyperconjugation only
C
both inductive effect and hyperconjugation
D
electromeric effect only
Answer :
both inductive effect and hyperconjugation
175.
Arrange the following carbocations in decreasing order of stability.
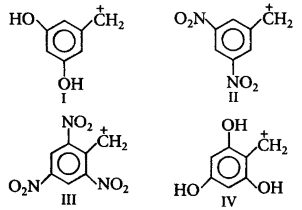
A
II > I > III > IV
B
III > IV > I > II
C
II > III > I > IV
D
IV > I > II > III
Answer :
IV > I > II > III
176. The hybridization of carbon atoms in $$C-C$$ single bond of $$HC \equiv C - CH = C{H_2}\,{\text{is}}$$
A
$$s{p^3} - s{p^3}$$
B
$$s{p^2} - s{p^3}$$
C
$$sp - s{p^2}$$
D
$$s{p^3} - sp$$
Answer :
$$sp - s{p^2}$$
177. In Carius method of estimation of halogen, $$0.15\,g$$ of an organic compound gave $$0.12\,g$$ of $$AgBr.$$ What is the percentage of bromine in the compound?
A
$$68.08\% $$
B
$$34.04\% $$
C
$$42.1\% $$
D
$$50\% $$
Answer :
$$34.04\% $$
178. Which of the following is a false statement?
A
Free radicals, carbonium ions or carbanions are reaction intermediates
B
Reaction between methane and chlorine in presence of sunlight proceeds $$via$$ free radical
C
The electronegative atom in the carbon chain produces $$+I$$ effect
D
Homolytic fission of $$C - C$$ bonds gives free radicals
Answer :
The electronegative atom in the carbon chain produces $$+I$$ effect
179. Which of the following represents the given sequence of hybridisation of carbon atoms from left to right $$s{p^2},s{p^2},sp,sp?$$
A
$${H_2}C = CH - C \equiv CH$$
B
$$HC \equiv C - CH = C{H_2}$$
C
$${H_3}C - CH = CH - C{H_3}$$
D
$${H_2}C = CH - CH = C{H_2}$$
Answer :
$${H_2}C = CH - C \equiv CH$$
180. Freshly prepared solution of sodium nitroprusside is added to the sodium extract. Appearance of a deep violet colour indicates the presence of
A
nitrogen
B
sulphur
C
both nitrogen and sulphur
D
halogen
Answer :
sulphur