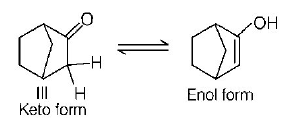
151.
Identify the correct order of boiling points of the following compounds;
$$\mathop {C{H_3}C{H_2}C{H_2}C{H_2}OH}\limits_{\left( {\text{i}} \right)} ,$$ $$\mathop {C{H_3}C{H_2}C{H_2}CHO}\limits_{\left( {{\text{ii}}} \right)} ,$$ $$\mathop {C{H_3}C{H_2}C{H_2}COOH}\limits_{\left( {{\text{iii}}} \right)} $$
A
(i) > (ii) > (iii)
B
(iii) > (i) > (ii)
C
(i) > (iii) > (ii)
D
(iii) > (ii) > (i)
Answer :
(iii) > (i) > (ii)
152.
Which among the given molecules can exhibit tautomerism?
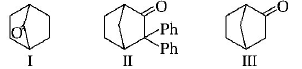
A
III Only
B
Both I and III
C
Both I and II
D
Both II and III
Answer :
III Only
153. $$1.6\,g$$ of an organic compound gave $$2.6\,g$$ of magnesium pyrophosphate. The percentage of phosphorus in the compound is
A
$$45.38\% $$
B
$$54.38\% $$
C
$$37.76\% $$
D
$$19.02\% $$
Answer :
$$45.38\% $$
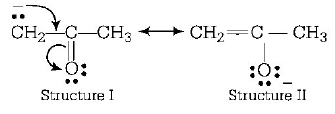
154.
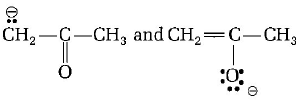 are
are
A
resonating structures
B
tautomers
C
geometrical isomers
D
optical isomers
Answer :
resonating structures
155. Which of the following is an electrophilic reagent?
A
$${H_2}O$$
B
$$N{H_3}$$
C
$$O{H^ - }$$
D
$$NO_2^ + $$
Answer :
$$NO_2^ + $$
156. What is the decreasing order of strength of the bases $$O{H^ - },NH_2^ - ,HC \equiv {C^ - }$$ and $$C{H_3}CH_2^ - ?$$
A
$$C{H_3}CH_2^ - > NH_2^ - > HC \equiv {C^ - } > O{H^ - }$$
B
$$HC \equiv {C^ - } > C{H_3}CH_2^ - > NH_2^ - > O{H^ - }$$
C
$$O{H^ - } > NH_2^ - > HC \equiv {C^ - } > C{H_3}CH_2^ - $$
D
$$NH_2^ - > HC \equiv {C^ - } > O{H^ - } > C{H_3}CH_2^ - $$
Answer :
$$C{H_3}CH_2^ - > NH_2^ - > HC \equiv {C^ - } > O{H^ - }$$
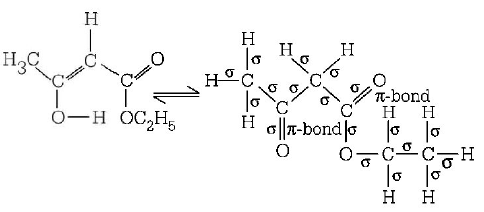
157.
The enolic form of ethyl acetoacetate as below has
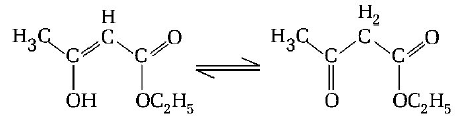
A
18 sigma bonds and 2 $$pi$$ - bonds
B
16 sigma bonds and 1 $$pi$$ -bond
C
9 sigma bonds and 2 $$pi$$ -bonds
D
9 sigma bonds and 1 $$pi$$ -bond
Answer :
18 sigma bonds and 2 $$pi$$ - bonds
158.
Arrange the following $$(w, x, y, z)$$ in decreasing order of their boiling points :
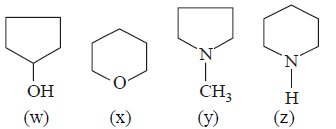
A
$$w > x > z > y$$
B
$$w > x > y > z$$
C
$$w > z > y > x$$
D
$$w > z > x > y$$
Answer :
$$w > z > x > y$$
159. Which of the following is a characteristic feature of a free radical?
A
It has a positive charge.
B
It has a negative charge.
C
It has all paired electrons.
D
It has an unpaired electron.
Answer :
It has an unpaired electron.
160. Molecule in which the distance between the two adjacent carbon atoms is largest is
A
Ethane
B
Ethene
C
Ethyne
D
Benzene
Answer :
Ethane