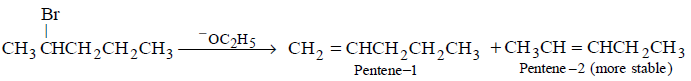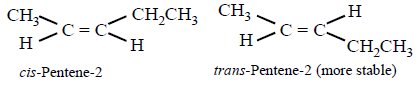201. Alkyl halides are formed when thionyl chloride and ________ are refluxed in presence of pyridine. The order of reactivity $$\left( {{3^ \circ } > {2^ \circ } > {1^ \circ }} \right)$$ is due to $$+I$$ effect of the alkyl group which ________ the polarity of $$C-X$$ bond.
A
acids, decreases
B
alcohols, increases
C
aldehydes, changes
D
ketones, decreases
Answer :
alcohols, increases
202. 2 - Bromopentane is heated with potassium ethoxide in ethanol. The major product obtained is
A
2 - Ethoxypentane
B
Pentene - 1
C
$$cis$$ - Pentene - 2
D
$$trans$$ - Pentene - 2
Answer :
$$trans$$ - Pentene - 2
203.
The order of reactivity of following alcohols with halogen acids is __________.
\[\begin{align}
& \left( \text{i} \right)C{{H}_{3}}C{{H}_{2}}-C{{H}_{2}}-OH \\
& \left( \text{ii} \right)C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
C{{H}_{3}}
\end{smallmatrix}}{\mathop{-CH-}}\,OH \\
& \left( \text{iii} \right)C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,OH \\
\end{align}\]
A
(i) > (ii) > (iii)
B
(iii) > (ii) > (i)
C
(ii) > (i) > (iii)
D
(i) > (iii) > (ii)
Answer :
(iii) > (ii) > (i)
204.
Which of the following halides is not correct according to the name and classification?
\[\left( \text{i} \right)C{{H}_{3}}C{{H}_{2}}C{{\left( C{{H}_{3}} \right)}_{2}}C{{H}_{2}}I\]
1-Iodo-2, 2-dimethylbutane, Primary haloalkane
\[\left( \text{ii} \right){{\left( C{{H}_{3}} \right)}_{2}}CHCH\left( Cl \right)C{{H}_{3}}\]
2-Chloro-3-methylbutane, Secondary haloalkane
\[\left( \text{iii} \right)C{{H}_{3}}C\left( Cl \right)\left( {{C}_{2}}{{H}_{5}} \right)C{{H}_{2}}C{{H}_{3}}\]
2-Chloro-2-ethylbutane, Secondary haloalkane
\[\left( \text{iv} \right)C{{H}_{3}}-C{{H}_{2}}\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-CH}}\,\overset{\begin{smallmatrix}
Cl\, \\
|\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,\] \[C{{H}_{2}}-C{{H}_{3}}\]
3-Chloro-4-methylhexane, Secondary haloalkane
A
(i)
B
(ii)
C
(iii)
D
(iv)
Answer :
(iii)
205. Which of the following reactions follows Markovnikov's rule?
A
$${C_2}{H_4} + HBr$$
B
$${C_3}{H_6} + C{l_2}$$
C
$${C_3}{H_6} + HBr$$
D
$${C_3}{H_6} + B{r_2}$$
Answer :
$${C_3}{H_6} + HBr$$
206. Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated $$HCl$$ at room temperature?
A
\[C{{H}_{3}}C{{H}_{2}}-C{{H}_{2}}-OH\]
B
\[C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
C{{H}_{3}}\,
\end{smallmatrix}}{\mathop{-CH-}}\,OH\]
C
\[C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
C{{H}_{3}}\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}OH\]
D
\[C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
| \\
\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,OH\]
Answer :
\[C{{H}_{3}}C{{H}_{2}}\underset{\begin{smallmatrix}
| \\
\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,OH\]
207.
Arrange the following compounds in increasing order of their boiling points.
$$\left( {\text{i}} \right)$$ 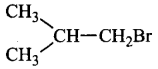
\[\begin{align}
& \left( \text{ii} \right)C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Br \\
& \left( \text{iii} \right){{H}_{3}}C\underset{\begin{smallmatrix}
| \\
\,Br
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,C{{H}_{3}} \\
\end{align}\]
A
(ii) < (i) < (iii)
B
(i) < (ii) < (iii)
C
(iii) < (i) < (ii)
D
(iii) < (ii) < (i)
Answer :
(iii) < (i) < (ii)
208. Which of the following molecules has highest dipole moment?
A
$$C{H_3}Cl$$
B
$$C{H_2}C{l_2}$$
C
$$CHC{l_3}$$
D
$$CC{l_4}$$
Answer :
$$C{H_3}Cl$$
209. Which of the following statements is not correct about $${S_N}2$$ reactions of alkyl halides?
A
Nucleophile attacks the carbon from the side opposite to where the leaving group is attached.
B
The bond formation and bond breaking take place in one step.
C
The rate of reaction depends upon the concentration of nucleophile.
D
$${S_N}2$$ mechanism is predominant in tertiary alkyl halides.
Answer :
$${S_N}2$$ mechanism is predominant in tertiary alkyl halides.
210.
Among the isomers of the one which is $${C_5}{H_{11}}Cl,$$ the one which is chiral is
(i) 2, 2-Dimethy-1-chloropropane
(ii) 2-Chloropentane
(iii) 2-Methyl- 2-chlorobutane
(iv) 3-Chloropentane
A
(i) and (ii) only
B
(i), (ii) and (iii) only
C
(i) and (iii) only
D
(ii) only
Answer :
(ii) only