192. When $$C{H_3}C{H_2}CHC{l_2}$$ is treated with $$NaN{H_2},$$ the product formed is
A
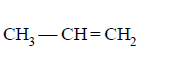
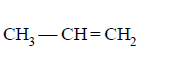
B
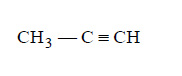
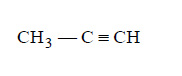
C
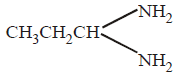
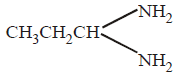
D
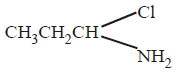
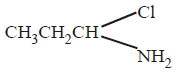
Answer :
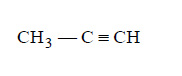
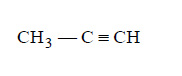
193. Chloroform is kept in dark coloured bottles because
A
it reacts with clear glass
B
it undergoes chlorination in transparent glass bottles
C
it is oxidised to poisonous gas, phosgene in sunlight
D
it starts burning when exposed to sunlight
Answer :
it is oxidised to poisonous gas, phosgene in sunlight
194.
The increasing order of the reactivity of the following halides for the $${S_N}1$$ reaction is
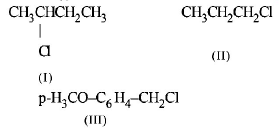
A
(III) < (II) < (I)
B
(II) < (I) < (III)
C
(I) < (III) < (II)
D
(II) < (III) < (I)
Answer :
(II) < (I) < (III)
195. Which of the following is a primary halide?
A
$$iso$$ - Propyl iodide
B
$$sec$$ - Butyl iodide
C
$$tert$$ - Butyl bromide
D
$$neo$$ - Hexyl chloride
Answer :
$$neo$$ - Hexyl chloride
196.
Consider the following reaction :
\[{{C}_{6}}{{H}_{5}}\underset{\begin{smallmatrix}
| \\
H\,
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,Br+{{H}_{2}}O\to \] \[HO\underset{\begin{smallmatrix}
| \\
H\,
\end{smallmatrix}}{\overset{\begin{smallmatrix}
\,\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,{{C}_{6}}{{H}_{5}}+HBr\]
The reaction proceeds with $$98\% $$ racemisation. The reaction may follow
A
$${S_N}1$$ mechanism
B
$${S_N}2$$ mechanism
C
$$E1$$ mechanism
D
$$E2$$ mechanism
Answer :
$${S_N}1$$ mechanism
197. For the compounds $$C{H_3}Cl,C{H_3}Br,C{H_3}I$$ and $$C{H_3}F,$$ the correct order of increasing $$C–X$$ bond length is :
A
$$C{H_3}F < C{H_3}Cl < C{H_3}Br < C{H_3}I$$
B
$$C{H_3}F < C{H_3}Br < C{H_3}Cl < C{H_3}I$$
C
$$C{H_3}F < C{H_3}I < C{H_3}Br < C{H_3}Cl$$
D
$$C{H_3}Cl < C{H_3}Br < C{H_3}F < C{H_3}I$$
Answer :
$$C{H_3}F < C{H_3}Cl < C{H_3}Br < C{H_3}I$$
198.
Match the reactions given in column I with the type of reaction mentioned in column II and mark the appropriate choice.
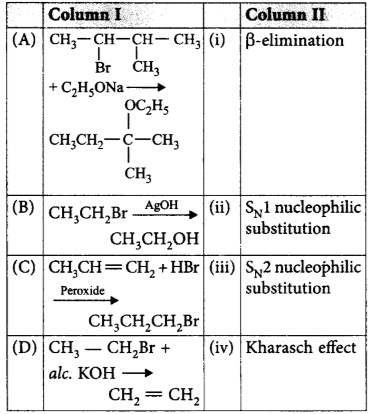
A
A - iv, B - i, C - ii, D - iii
B
A - ii, B - iii, C - iv, D - i
C
A - i, B - ii, C - iv, D - iii
D
A - iii, B - i, C - ii, D - iv
Answer :
A - ii, B - iii, C - iv, D - i
199. The missing reagents $${R_1}$$ and $${R_2}$$ in the following series of reactions are \[C{{H}_{3}}C{{H}_{2}}Br\xrightarrow{{{R}_{1}}}\left[ \,\,\,\, \right]\xrightarrow{{{R}_{2}}}C{{H}_{3}}\overset{-}{\mathop{C}}\,H{{P}^{+}}P{{h}_{3}}\]
A
$$PhLi$$ and $$P{h_3}P$$ respectively
B
$$P{h_3}P$$ and $$PhLi$$ respectively
C
$$P{h_3}P$$ and $${C_2}{H_5}ONa$$ respectively
D
Either (B) or (C)
Answer :
Either (B) or (C)
200.
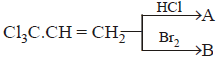
Which of the following is correct ?
A
$$A$$ on reaction with $$aq.\,KOH$$ gives $$HOC{H_2}C{H_2}COOK$$
B
$$B$$ can be resolved into $$d–$$ and $$l-$$ forms
C
Both (A) and (B)
D
Neither (A) nor (B)
Answer :
Both (A) and (B)
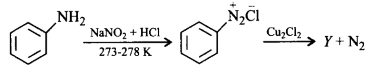
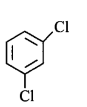
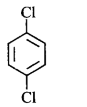
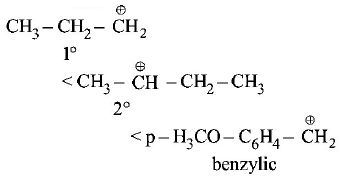
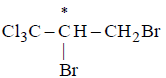 has chiral centre and can be resolved into $$d-$$ and $$l-$$ form.
has chiral centre and can be resolved into $$d-$$ and $$l-$$ form.