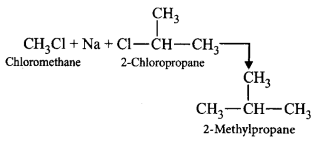
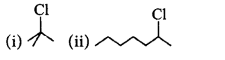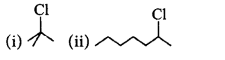181. On treating a mixture of two alkyl halides with sodium metal in dry ether, 2-methylpropane was obtained. The alkyl halides are
A
2-chloropropane and chloromethane
B
2-chloropropane and chloroethane
C
chloromethane and chloroethane
D
chloromethane and 1-chloropropane
Answer :
2-chloropropane and chloromethane
182.
Which of the following reactions will give the major and minor products?
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\, \\
Br\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH-}}\,C{{H}_{3}}\xrightarrow[\text{heat}]{alc.\,KOH}\]
$$C{H_3} - \mathop {CH = CH}\limits_{\left( A \right)} - C{H_3} + C{H_3}$$ $$ - \mathop {C{H_2} - CH}\limits_{\left( B \right)} = C{H_2}$$
A
$$(A)$$ is major product and $$(B)$$ is minor product.
B
$$(A)$$ is minor product and $$(B)$$ is major product.
C
Both $$(A)$$ and $$(B)$$ are major products.
D
Only $$(B)$$ is formed and $$(A)$$ is not formed.
Answer :
$$(A)$$ is major product and $$(B)$$ is minor product.
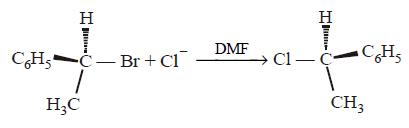
183. In a nucleophilic substitution reaction : \[R-Br+C{{l}^{-}}\xrightarrow{DMF}R-Cl+B{{r}^{-}},\] Which one of the following undergoes complete inversion of configuration ?
A
$${C_6}{H_5}CH{C_6}{H_5}Br$$
B
$${C_6}{H_5}C{H_2}Br$$
C
$${C_6}{H_5}CHC{H_3}Br$$
D
$${C_6}{H_5}CC{H_3}{C_6}{H_5}Br$$
Answer :
$${C_6}{H_5}CHC{H_3}Br$$
184. Which of the following will give enantiomeric pair on reaction with water due to presence of asymmetric carbon atom?
A
\[{{C}_{2}}{{H}_{5}}\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\, \\
{{C}_{2}}{{H}_{5}}\,\,\,
\end{smallmatrix}}{\overset{\begin{smallmatrix}
{{C}_{2}}{{H}_{5}}\, \\
|\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{-\,\,C-Br}}}\,\]
B
\[{{C}_{2}}{{H}_{5}}\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\, \\
C{{H}_{3}}\,\,\,
\end{smallmatrix}}{\overset{\begin{smallmatrix}
{{C}_{2}}{{H}_{5}} \\
|\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{-\,\,C-Cl}}}\,\]
C
\[{{C}_{2}}{{H}_{5}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
H \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,I\]
D
\[C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\, \\
{{C}_{2}}{{H}_{5}}\,\,\,
\end{smallmatrix}}{\overset{\begin{smallmatrix}
C{{H}_{3}} \\
|\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{-\,\,C-Br}}}\,\]
Answer :
\[{{C}_{2}}{{H}_{5}}\underset{\begin{smallmatrix}
| \\
\,\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\overset{\begin{smallmatrix}
H \\
|
\end{smallmatrix}}{\mathop{-C-}}}\,I\]
185. Tertiary butyl bromide on reaction with sodium methoxide mainly gives
A
$$tert$$ - butyl ethyl ether
B
2 - methylpropene
C
propene
D
$$iso$$ - propyl alcohol
Answer :
2 - methylpropene
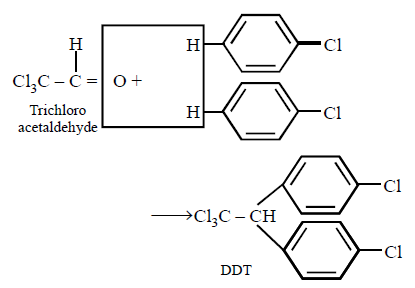
186.
Chlorobenzne reacts with trichloro acetaldehyde in the presence of $${H_2}S{O_4}.$$
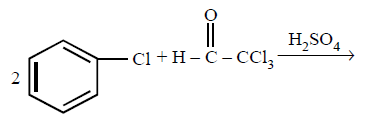
The major product formed is :
A
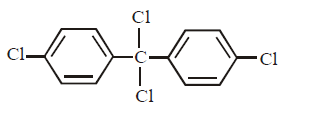

B
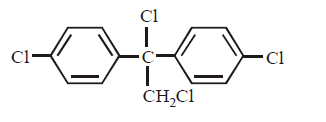

C
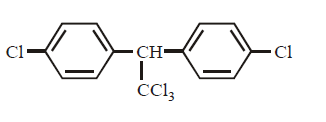

D
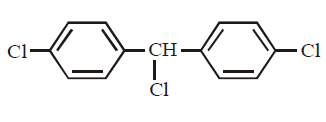

Answer :


187. The major product formed when 1, 1, 1-trichloro-propane is treated with aqueous potassium hydroxide is :
A
Propyne
B
1-Propanol
C
2-Propanol
D
Propionic acid
Answer :
Propionic acid
188. Pure $$\left( S \right) - C{H_3}C{H_2}CHBrC{H_3}$$ is subjected to monobromination to form several isomers of $${C_4}{H_8}B{r_2}.$$ Which of the following is not produced?
A
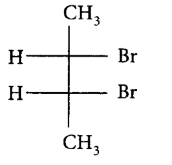

B
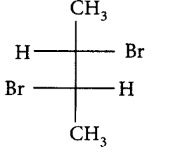

C
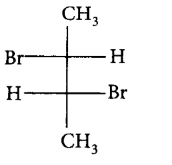

D
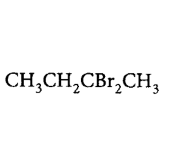
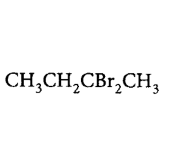
Answer :
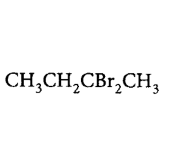
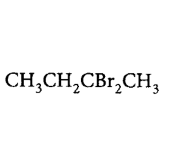
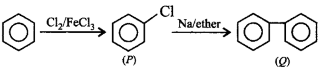
189.
The end product $$(Q)$$ in the following sequence of reactions is
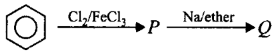
A
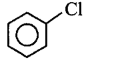

B
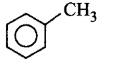

C
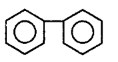

D
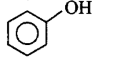

Answer :


190.
In the following pairs of halogen compounds, which compound undergoes faster $${S_N}1$$ reaction?
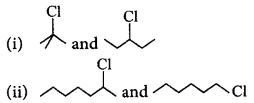
A
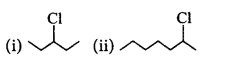
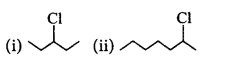
B
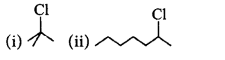
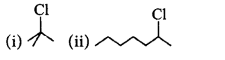
C
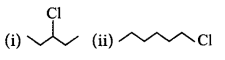
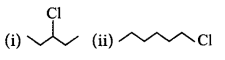
D
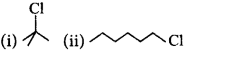

Answer :