171. The fire extinguisher 'pyrene' contains
A
carbon dioxide
B
carbon disulphide
C
carbon tetrachloride
D
chloroform
Answer :
carbon tetrachloride
172.
Which is the correct increasing order of boiling points of the following compounds?
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
A
Bromobenzene < 1-Brornobutane < 1-Bromopropane < 1-Bromoethane
B
Bromobenzene < 1-Bromoethane < 1-Bromopropane < 1-Bromobutane
C
1-Bromopropane < 1-Bromobutane < 1-Bromoethane < Bromobenzene
D
1-Brornoethane < 1-Brornopropane < 1-Bromobutane < Bromobenzene
Answer :
1-Brornoethane < 1-Brornopropane < 1-Bromobutane < Bromobenzene
173.
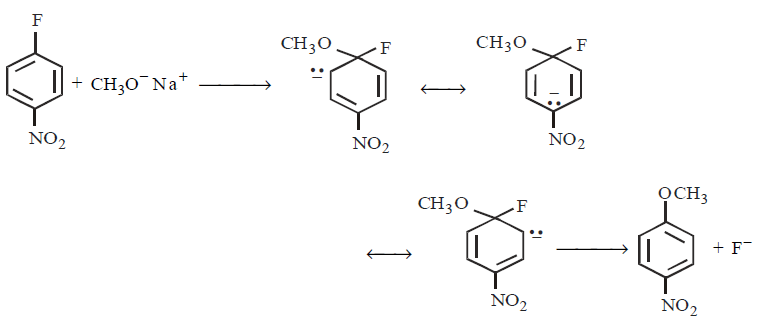
Which of the following is (are) true concerning the intermediate in the addition-elimination mechanism of the following reaction ?
$$A =$$ The intermediate is aromatic, $$B =$$ The intermediate is a resonance stabilised anion, $$C =$$ Electron withdrawing groups on the benzene ring stabilises the intermediate
A
only $$A$$
B
only $$B$$
C
$$A$$ and $$C$$
D
$$B$$ and $$C$$
Answer :
$$B$$ and $$C$$
174.
Match the column I with column II and mark the appropriate choice.
Column I
Column II
a.
Carbon tetrachloride
1.
Paint remover
b.
Methylene chloride
2.
Refrigerators and air conditioners
c.
DDT
3.
Fire-extinguisher
d.
Freons
4.
Non-biodegradable insecticide
A
a - 2, b - 3, c - 1, d - 4
B
a - 4, b - 3, c - 2, d - 1
C
a - 1, b - 2, c - 3, d - 4
D
a - 3, b - 1, c - 4, d - 2
Answer :
a - 3, b - 1, c - 4, d - 2
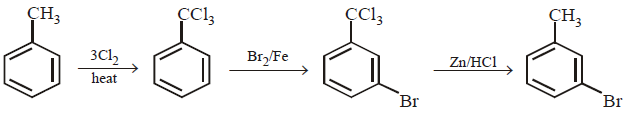
175.
The compound \[{{C}_{7}}{{H}_{8}}\xrightarrow{3C{{l}_{2}}/\Delta }A\xrightarrow{B{{r}_{2}}/Fe}B\xrightarrow{Zn/HCl}C\]
The compound $$C$$ is
A
$$o$$ - Bromotoluene
B
$$m$$ - Bromotoluene
C
$$p$$ - Bromotoluene
D
3 - Bromo -2, 4, 6 - trichlorotoluene
Answer :
$$m$$ - Bromotoluene
176.
The reaction is described as
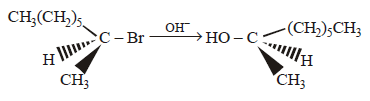
A
$${S_E}2$$
B
$${S_N}1$$
C
$${S_N}2$$
D
$${S_N}0$$
Answer :
$${S_N}2$$
177. Elimination of bromine from 2-bromobutane results in the formation of
A
equimolar mixture of 1and 2-butene
B
predominantly 2-butene
C
predominantly 1-butene
D
predominantly 2-butyne
Answer :
predominantly 2-butene
178.
Which of the following structures is enantiomeric with the molecule $$\left( I \right)$$ given below?

A
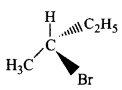

B
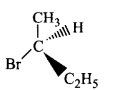

C
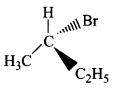

D
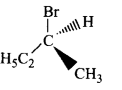

Answer :


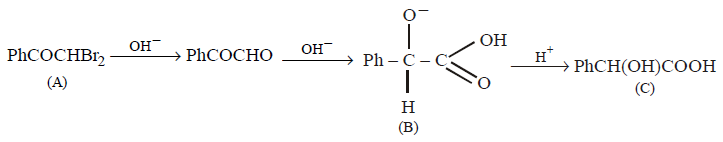
179.
\[PhCOCHB{{r}_{2}}\xrightarrow{O{{H}^{-}}}A\xrightarrow{O{{H}^{-}}}B\xrightarrow{{{H}^{+}}}C\]
The compound $$C$$ is –
A
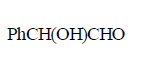
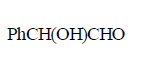
B
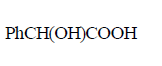
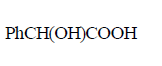
C


D
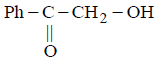
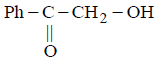
Answer :
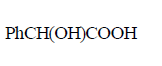
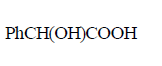
180. An alkyl halide, $$RX$$ reacts with $$KCN$$ to give propane nitrile. $$RX$$ is
A
$${C_3}{H_7}Br$$
B
$${C_4}{H_9}Br$$
C
$${C_2}{H_5}Br$$
D
$${C_5}{H_{11}}Br$$
Answer :
$${C_2}{H_5}Br$$