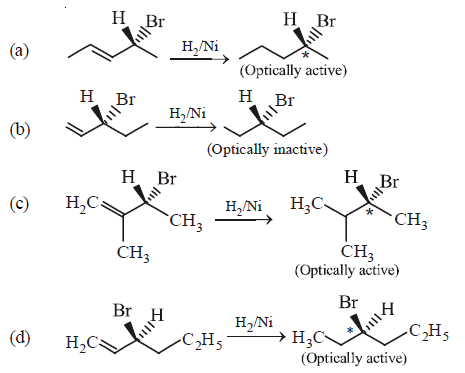
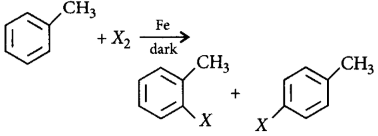
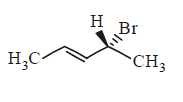
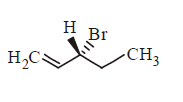
161. Compound that on hydrogenation produces optically inactive compound is
A

B

C
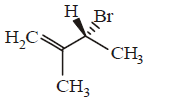

D
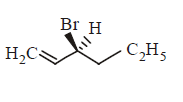

Answer :


163. The ease of dehydrohalogenation of alkyl halide with alcoholic $$KOH$$ is
A
$${3^ \circ } < {2^ \circ } < {1^ \circ }$$
B
$${3^ \circ } > {2^ \circ } > {1^ \circ }$$
C
$${3^ \circ } < {2^ \circ } > {1^ \circ }$$
D
$${3^ \circ } > {2^ \circ } < {1^ \circ }$$
Answer :
$${3^ \circ } > {2^ \circ } > {1^ \circ }$$
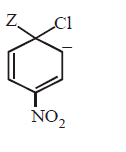
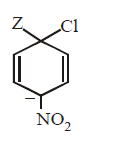
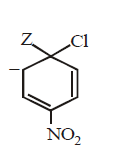
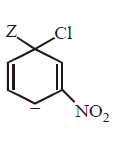
165.
The IUPAC name of the compoun 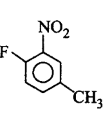 is
is
A
1-fluoro-4- methyl- 2-nitrobenzene
B
4-fluoro-1-methyl-3-nitrobenzene
C
4-methyl-1- fluoro- 2-nitrobenzene
D
2-fluoro-5-methyl-1-nitrobenzene
Answer :
1-fluoro-4- methyl- 2-nitrobenzene
166.
Which reagent will you use for the following reaction?
$$C{H_3}C{H_2}C{H_2}C{H_3} \to $$ $$C{H_3}C{H_2}C{H_2}C{H_2}Cl \,+ $$ $$C{H_3}C{H_2}CHClC{H_3}$$
A
$$C{l_2}/UV$$ light
B
$$NaCl + {H_2}S{O_4}$$
C
$$C{l_2}$$ gas in dark
D
$$C{l_2}$$ gas in the presence of iron in dark
Answer :
$$C{l_2}/UV$$ light
167. Which of the following is the most reactive towards nucleophilic substitution reaction?
A
$$ClC{H_2} - CH = C{H_2}$$
B
$$C{H_2} = CH - Cl$$
C
$$C{H_3}CH = CH - Cl$$
D
$${C_6}{H_5}Cl$$
Answer :
$$ClC{H_2} - CH = C{H_2}$$
168. Identify the products $$X$$ and $$Y$$ in the given reaction, \[C{{H}_{3}}\underset{\begin{smallmatrix} |\,\,\,\,\, \\ Br\,\,\,\, \end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}}+Mg\] \[\xrightarrow{\text{Dry}\,\,\text{ether}}X\xrightarrow{{{D}_{2}}O}Y\]
A
\[X=C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
Br\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}Mg,\] \[Y=C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
B
\[X=C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
\,MgBr
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}},\] \[Y=C{{H}_{3}}-\underset{\begin{smallmatrix}
|\,\,\,\, \\
D\,\,\,\,
\end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
C
\[X=C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
\,MgBr
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}},\] \[Y=C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OD\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}}\]
D
\[X=C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
Br\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{2}}Mg,\] \[Y=C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
OH\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}}\]
Answer :
\[X=C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
\,MgBr
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}},\] \[Y=C{{H}_{3}}-\underset{\begin{smallmatrix}
|\,\,\,\, \\
D\,\,\,\,
\end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
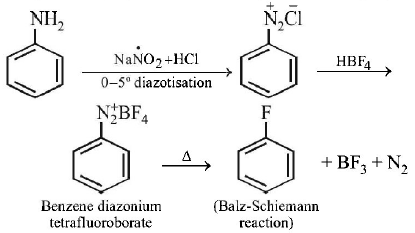
169. Fluorobenzene $$\left( {{C_6}{H_5}F} \right)$$ can be synthesized in the laboratory
A
by direct fluorination of benzene with $${F_2}$$ gas
B
by reacting bromobenzene with $$NaF$$ solution
C
by heating phenol with $$HF$$ and $$KF$$
D
from aniline by diazotisation followed by heating the
diazonium salt with $$HB{F_4}$$
Answer :
from aniline by diazotisation followed by heating the
diazonium salt with $$HB{F_4}$$
170.
$$C{H_3}Br + N{u^ - } \to C{H_3} - Nu + B{r^ - }$$
The decreasing order of the rate of the above reaction with nucleophiles $$\left( {N{u^ - }} \right)A$$ to $$D$$ is $$\left[ {N{u^ - } = \left( A \right)Ph{O^ - },\left( B \right)Ac{O^ - },\left( C \right)H{O^ - },\left( D \right)C{H_3}{O^ - }} \right]$$
A
$$A > B > C > D$$
B
$$B > D > C > A$$
C
$$D > C > A > B$$
D
$$D > C > B > A$$
Answer :
$$D > C > A > B$$