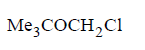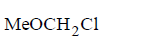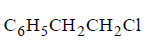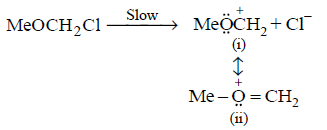151.
\[C{{H}_{3}}\underset{\begin{smallmatrix}
|\,\,\,\,\, \\
Br\,\,\,\,
\end{smallmatrix}}{\mathop{-CH-}}\,C{{H}_{3}}\xrightarrow{alc.\,KOH}X\] \[\xrightarrow[\text{Peroxide}]{HBr}Y\xrightarrow[\text{Acetone}]{NaI}Z\]
In the given reaction what will be the final product?
A
$$C{H_3}C{H_2}C{H_2}I$$
B
$$C{H_3}CHIC{H_2}I$$
C
$$C{H_3}C{H_2}C{H_2}C{H_2}C{H_3}$$
D
$$C{H_3}C{H_2}CH{I_2}$$
Answer :
$$C{H_3}C{H_2}C{H_2}I$$
152. The reaction of toluene with chlorine in presence of ferric chloride gives predominantly :
A
benzoyl chloride
B
$$m$$ - chlorotoluene
C
benzyl chloride
D
$$o$$ - and $$p$$ - chlorotoluene
Answer :
$$o$$ - and $$p$$ - chlorotoluene
154. Which of the following compounds has the highest boiling point?
A
$$C{H_3}C{H_2}C{H_2}Cl$$
B
$$C{H_3}C{H_2}C{H_2}C{H_2}Cl$$
C
$$C{H_3}CH\left( {C{H_3}} \right)C{H_2}Cl$$
D
$${\left( {C{H_3}} \right)_3}CCl$$
Answer :
$$C{H_3}C{H_2}C{H_2}C{H_2}Cl$$
155. Which of the following haloalkanes reacts with aqueous $$KOH$$ most easily?
A
1-Bromobutane
B
2-Bromobutane
C
2-Bromo-2-methylpropane
D
2-Chlorobutane
Answer :
2-Bromo-2-methylpropane
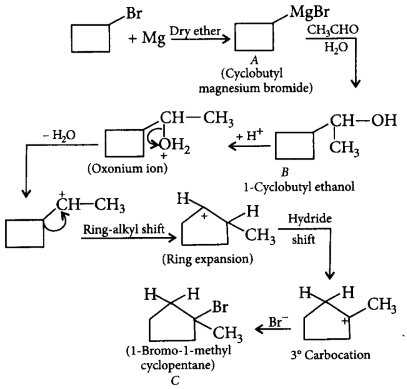
156. Cyclobutyl bromide on treatment with magnesium in dry ether forms an organometallic compound $$(A).$$ The organometallic compound reacts with ethanal to give an alcohol $$(B)$$ after mild acidification. Prolonged treatment of alcohol $$(B)$$ with an equivalent amount of $$HBr$$ gives $$(C).$$ What will be the product $$'C'?$$
A
1-Chloro-1-ethylcyclopentane
B
1-Bromo-1-methylcyclopentane
C
3-Bromo-2-methylcyclopentane
D
None of these
Answer :
1-Bromo-1-methylcyclopentane
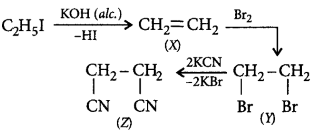
157.
Identify $$(Z)$$ in the following reaction series,
\[{{C}_{2}}{{H}_{5}}I\xrightarrow[KOH]{\text{Alcoholic}}\left( X \right)\xrightarrow{B{{r}_{2}}}\] \[\left( Y \right)\xrightarrow{KCN}\left( Z \right)\]
A
\[C{{H}_{3}}-C{{H}_{2}}-CN\]
B
\[\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
CN\,\,\,\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{C{{H}_{2}}\,\,-}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\, \\
CN\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,\]
C
\[\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
Br\,\,\,\,\,\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{C{{H}_{2}}\,\,-}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\, \\
CN\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,\]
D
\[\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
Br\,\,\,\,\,\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH\,\,=}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\, \\
CN\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{CH}}\,\]
Answer :
\[\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
CN\,\,\,\,\,\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{C{{H}_{2}}\,\,-}}\,\underset{\begin{smallmatrix}
|\,\,\,\,\,\,\,\, \\
CN\,\,\,\,\,\,
\end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,\]
158. Which of the following is not correctly matched with its IUPAC name?
A
$$CH{F_2}CBrClF:$$ 1-Bromo-1-chloro-1, 2, 2-trifluoroethane
B
$${\left( {CC{l_3}} \right)_3}CCl:$$ 2-(Trichloromethyl)-1, 1, 1, 2, 3, 3, 3-heptachloropropane
C
$$C{H_3}C{\left( {p - Cl{C_6}{H_4}} \right)_2}CH\left( {Br} \right)C{H_3}:$$ 2-Bromo-3, 3-$$bis$$ ( 4-chlorophenyl ) butane
D
$$o - Br{C_6}{H_4}CH\left( {C{H_3}} \right)C{H_2}C{H_3}:$$ 2-Bromo-1-methylpropylbenze
Answer :
$$o - Br{C_6}{H_4}CH\left( {C{H_3}} \right)C{H_2}C{H_3}:$$ 2-Bromo-1-methylpropylbenze
159. Which of the following is an example of $$vic$$ - dihalide?
A
Dichloromethane
B
1, 2-Dichloroethane
C
Ethylidene chloride
D
Allyl chloride
Answer :
1, 2-Dichloroethane
160. Among the following, the molecule with the lowest dipole moment is
A
$$CHC{l_3}$$
B
$$C{H_3}Cl$$
C
$$C{H_2}C{l_2}$$
D
$$CC{l_4}$$
Answer :
$$CC{l_4}$$