111. Alkyl halides are immiscible in water though they are polar because
A
they react with water to give alcohols
B
they cannot form hydrogen bonds with water
C
$$C - X$$ bond cannot be broken easily
D
they are stable compounds and are not reactive
Answer :
they cannot form hydrogen bonds with water
112. Which one of the following is not correct order of boiling points of the alkyl/aryl halides?
A
$$CHC{l_3} > C{H_2}C{l_2}$$
B
$$C{H_3}{\left( {C{H_2}} \right)_3}Cl > C{H_3}{\left( {C{H_2}} \right)_2}Cl$$
C
$${\left( {C{H_3}} \right)_3}CCl > {\left( {C{H_3}} \right)_2}CHC{H_2}Cl$$
D
$$C{H_3}{\left( {C{H_2}} \right)_3}Cl > C{H_3}C{H_2}CHClC{H_3}$$
Answer :
$${\left( {C{H_3}} \right)_3}CCl > {\left( {C{H_3}} \right)_2}CHC{H_2}Cl$$
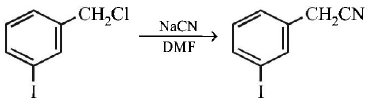
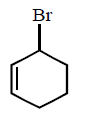
113.
The structure of the major product formed in the following
reaction
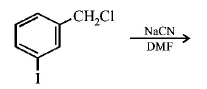
is
A
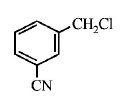

B
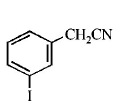

C
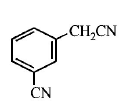

D
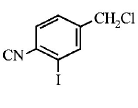

Answer :


114. The order of reactivity of the given haloalkanes towards nucleophile is :
A
$$RI > RBr > RCl$$
B
$$RCl > RBr > RI$$
C
$$RBr > RCl > RI$$
D
$$RBr > RI > RCl$$
Answer :
$$RI > RBr > RCl$$
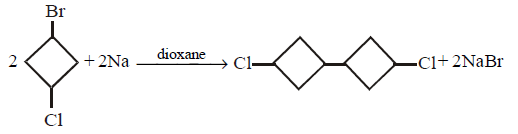
115. The product of 1 - bromo - 3 - chloro cyclobutane with $$Na$$ in presence of dioxane
A


B
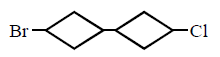

C
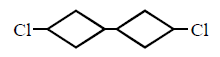

D
Answer :


116. Among the choices of alkyl bromide, the least reactive bromide in $${S_N}2$$ reaction is
A
1-bromopentane
B
2-bromo-2-methylbutane
C
1-bromo-3-methylbutane
D
1-bromo-2-methylbutane
Answer :
2-bromo-2-methylbutane
117. $$0.0852\,g$$ of an organic halide $$(A)$$ when dissolved in $$2.0\,g$$ of camphor, the melting point of the mixture was found to be $${167^ \circ }C.$$ Compound $$(A)$$ when heated with sodium gives a gas $$(B).$$ $$280\,mL$$ of gas $$(B)$$ at $$STP$$ weighs $$0.375\,g.$$ What would be $$'A'$$ in the whole process? ( $${K_f}$$ for camphor $$ = 40,m.pt.$$ of camphor $$ = {179^ \circ }C.$$ )
A
$${C_2}{H_5}Br$$
B
$$C{H_3}I$$
C
$${\left( {C{H_3}} \right)_2}CHI$$
D
$${C_3}{H_7}Br$$
Answer :
$$C{H_3}I$$
118. Bromination of methane in presence of sunlight is a
A
nucleophilic substitution
B
free radical substitution
C
electrophilic substitution
D
nucleophilic addition
Answer :
free radical substitution
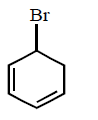
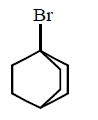
120. Which of the following has the weakest carbon - chlorine bond ?
A
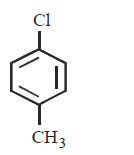

B


C
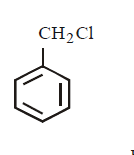

D

Answer :