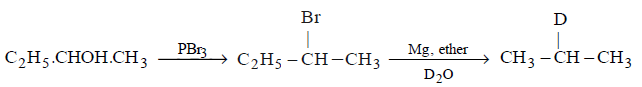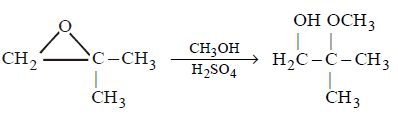171.
Match the column I with column II and mark the appropriate choice.
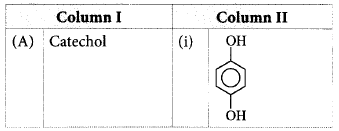
.PNG)
A
A - ii, B - iii, C - iv, D - i
B
A - i, B - ii, C - iii, D - iv
C
A - iv, B - iii, C - ii, D - i
D
A - ii, B - iv, C - i, D - iii
Answer :
A - ii, B - iii, C - iv, D - i
172. An equimolar quantities of ethanol and propanol is heated with conc. $${H_2}S{O_4}.$$ The product formed is/are
A
$${C_2}{H_5}O{C_2}{H_5}$$
B
$${C_3}{H_7}O{C_3}{H_7}$$
C
$${C_2}{H_5}O{C_3}{H_7}$$
D
$${\text{all of these}}$$
Answer :
$${\text{all of these}}$$
173.
Which of the following reactions would convert 2 - butanol into deuterated compound
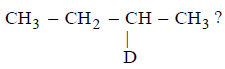
A


B
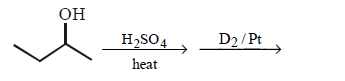
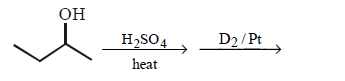
C


D


Answer :


174. Which one of the following compounds has the most acidic nature ?
A


B


C


D


Answer :


175. The major product obtained on interaction of phenol with sodium hydroxide and carbon dioxide is
A
salicylaldehyde
B
salicylic acid
C
phthalic acid
D
benzoic acid
Answer :
salicylic acid
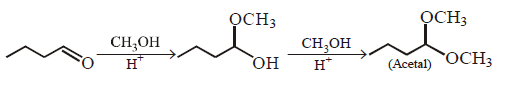
176.
Give the best conditions for this transformation :

A
$$C{H_3}OH,{H^ + }\left( {cat} \right),{\text{heat}}$$
B
$${H_2}O,{H^ + }\left( {cat.} \right),{\text{heat}}$$
C
$$Mg,{\text{ether}},C{H_3}OH$$
D
$$SOC{l_2},C{H_3}OH$$
Answer :
$$C{H_3}OH,{H^ + }\left( {cat} \right),{\text{heat}}$$
177. What happens when tertiary butyl alcohol is passed over heated copper at $${300^ \circ }C?$$
A
Secondary butyl alcohol is formed.
B
2-Methylpropene is formed.
C
1-Butene is formed.
D
Butanal is formed.
Answer :
2-Methylpropene is formed.
178.
In the following sequence of reactions,
 \[\xrightarrow{\text{Oleum}}P\xrightarrow[\left( \text{ii} \right){{H}^{+}}]{\left( \text{i} \right)NaOH}Q\]
\[\xrightarrow{\text{Oleum}}P\xrightarrow[\left( \text{ii} \right){{H}^{+}}]{\left( \text{i} \right)NaOH}Q\]
the compound $$Q$$ formed will be
A
aniline
B
phenol
C
benzaldehyde
D
benzene sulphonic acid
Answer :
phenol
179. An aromatic ether is not cleaved by $$HI$$ even at $$525\,K.$$ The compound is
A
$${C_6}{H_5}OC{H_3}$$
B
$${C_6}{H_5}O{C_6}{H_5}$$
C
$${C_6}{H_5}O{C_3}{H_7}$$
D
$${\text{Tetrahydrofuran}}$$
Answer :
$${C_6}{H_5}O{C_6}{H_5}$$
180.
What is $$X$$ in the following reaction ?
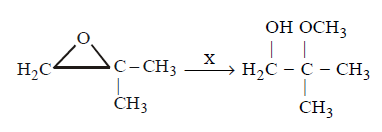
A
$$C{H_3}OH,{H_2}S{O_4}$$
B
\[C{{H}_{3}}OH,C{{H}_{3}}{{O}^{-}}\overset{+}{\mathop{N}}\,a\]
C
$${H_2}O/{H_2}S{O_4}\,\,{\text{followed by}}\,\,C{H_3}OH$$
D
$$C{H_3}MgBr/{\text{ether followed by}}\,\,{H_3}{O^ + }$$
Answer :
$$C{H_3}OH,{H_2}S{O_4}$$