241.
The correct order of strength of acidity of the following compounds is

A
(ii) > (i) > (iii) > (iv)
B
(i) > (ii) > (iii) > (iv)
C
(iv) > (iii) > (ii) > (i)
D
(iv) > (iii) > (i) > (ii)
Answer :
(iv) > (iii) > (i) > (ii)
242. IUPAC name of \[\underset{\begin{smallmatrix} | \\ \,\,\,\,\,\,\,\,\,\,C{{H}_{2}}OH \end{smallmatrix}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CH-C{{H}_{2}}C{{H}_{3}}}}\,\] is
A
3-propylbutan-1-ol
B
2-ethylpentan-1-ol
C
3-methyl hydroxyhexane
D
2-ethyl-2-propyl ethanol
Answer :
2-ethylpentan-1-ol
243.

\[\xrightarrow{\begin{smallmatrix}
\left( 1 \right)\,MeMgBr \\
\left( 2 \right)\,{{H}_{3}}{{O}^{+}}
\end{smallmatrix}}\left( C \right)\xrightarrow{NaB{{H}_{4}},EtOH}\left( D \right)\]
Product $$(D)$$ in above reaction is :
A


B


C


D


Answer :


244.
The reaction

Which of the following compounds will be formed?
A


B


C


D


Answer :


245. In order to get 2-hydroxybenzaldehyde from phenol, which of the following reagents is required ?
A
$${\left( {C{H_3}CO} \right)_2}O,{H_2}S{O_4}$$
B
$$CHC{l_3}/NaOH$$
C
$$C{O_2},NaOH$$
D
$$CC{l_4}/NaOH$$
Answer :
$$CHC{l_3}/NaOH$$
246.
Dehydration of alcohols by \[conc.\,{{H}_{2}}S{{O}_{4}}\] takes place according to following steps :
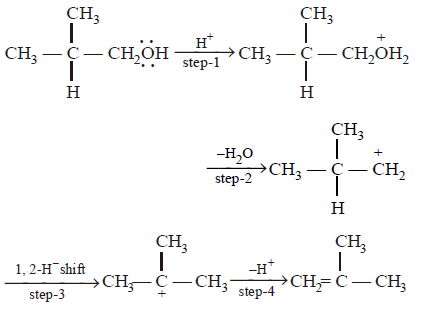
The slowest and fastest steps in the above reaction are
A
step 1 is slowest, while 3 is fastest.
B
step 2 is slowest, while 3 is fastest.
C
step 2 is slowest, while 4 is fastest.
D
all steps proceed at equal rate.
Answer :
step 2 is slowest, while 4 is fastest.
247.
The order of reactivity of the following alcohols towards $$conc.$$ $$HCl$$ is

A
I > II > III > IV
B
I > III > II > V
C
IV > III > II > I
D
IV > III > I > II
Answer :
IV > III > II > I
248.
\[C{{H}_{3}}-CH=C{{H}_{2}}\] \[\xrightarrow[\left( \text{ii} \right)\,NaB{{H}_{4}}]{\left( \text{i} \right)\,Hg{{\left( OAc \right)}_{2}}/{{H}_{2}}O}X+Na\] \[\to Y+C{{H}_{3}}Cl\to Z+HI\] \[\xrightarrow{{{0}^{\circ }}C}A+B\]
What are $$A$$ and $$B ?$$
A


B


C


D


Answer :


249.
Match the column I with column II and mark the appropriate choice.

A
A - i, B - ii, c - iii, D - iv
B
A - ii, B - iii, C - iv, D - i
C
A - iii, B - iv, C - i, D - ii
D
A - iv, B - iii, C - ii, D - i
Answer :
A - ii, B - iii, C - iv, D - i
250. Which one of the following compounds will not be soluble in sodium bicarbonate ?
A
2, 4, 6 - Trinitrophenol
B
Benzoic acid
C
$$o$$ - Nitrophenol
D
Benzene sulphonic acid
Answer :
$$o$$ - Nitrophenol





