231. On addition of cone. $${H_2}S{O_4}$$ to a chloride salt, colourless fumes are evolved but in case of iodide salt, violet fumes come out. This is because
A
$${H_2}S{O_4}$$ reduces $$HI$$ to $${I_2}$$
B
$$HI$$ is of violet colour
C
$$HI$$ gets oxidised to $${I_2}$$
D
$$HI$$ changes to $$HI{O_3}.$$
Answer :
$$HI$$ gets oxidised to $${I_2}$$
232. Pure nitrogen is prepared in the laboratory by heating a mixture of
A
$$N{H_4}OH + NaCl$$
B
$$N{H_4}N{O_3} + NaCl$$
C
$$N{H_4}Cl + NaOH$$
D
$$N{H_4}Cl + NaN{O_2}$$
Answer :
$$N{H_4}Cl + NaN{O_2}$$
233. Which one of the following reactions of xenon compounds is not feasible?
A
$$\,3Xe{F_4} + 6{H_2}O \to 2Xe + Xe + Xe{O_3} + 12HF + 1.5{O_2}$$
B
$$2Xe{F_2} + 2{H_2}O \to 2Xe + 4HF + {O_2}$$
C
$$Xe{F_6} + RbF \to Rb\left[ {Xe{F_7}} \right]$$
D
$$Xe{O_3} + 6HF \to Xe{F_6} + 3{H_2}O$$
Answer :
$$Xe{O_3} + 6HF \to Xe{F_6} + 3{H_2}O$$
234. Concentrated hydrochloric acid when kept in open air sometimes produces a cloud of white fumes. The explanation for it is that
A
oxygen in air reacts with the emitted $$HCl$$ gas to forma
cloud of chlorine gas
B
strong affinity of $$HCl$$ gas for moisture in air results in
forming of droplets of liquid solution which appears like
a cloudy smoke.
C
due to strong affinity for water, concentrated
hydrochloric acid pulls moisture of air towards itself.
This moisture forms droplets of water and hence the
cloud.
D
concentrated hydrochloric acid emits strongly smelling
$$HCl$$ gas all the time.
Answer :
oxygen in air reacts with the emitted $$HCl$$ gas to forma
cloud of chlorine gas
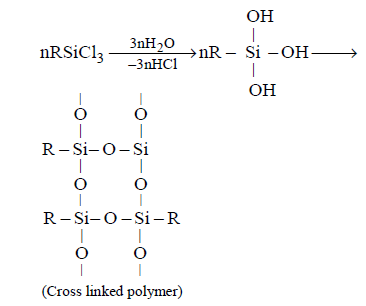
235. Among the following substituted silanes the one which will give rise to cross linked silicone polymer on hydrolysis is
A
$${R_4}Si$$
B
$${R_2}SiC{l_2}$$
C
$$RSiC{l_3}$$
D
$${R_3}SiCl$$
Answer :
$$RSiC{l_3}$$
236. Which of the following compounds does not exist ?
A
$$AsC{l_5}$$
B
$$SbC{l_3}$$
C
$$BiC{l_5}$$
D
$$SbC{l_5}$$
Answer :
$$BiC{l_5}$$
237. Which of the following compound does not exist?
A
$$NC{l_5}$$
B
$$As{F_5}$$
C
$$SbC{l_5}$$
D
$$P{F_5}$$
Answer :
$$NC{l_5}$$
238. Which of the following is not true regarding the nature of halides of boron?
A
Boron trihalides are covalent.
B
Boron trihalides are planar triangular with $$s{p^2}$$ hybridisation.
C
Boron trihalides act as Lewis acids.
D
Boron trihalides cannot be hydrolysed easily.
Answer :
Boron trihalides cannot be hydrolysed easily.
239. Which of the following statements is not true ?
A
$$N{O_2}$$ can be prepared by heating $$Pb{\left( {N{O_3}} \right)_2}.$$
B
$$N{O_2}$$ is red - brown gas.
C
$$N{O_2}$$ is diamagnetic.
D
$$N{O_2}$$ readily dimerises to $${N_2}{O_4}.$$
Answer :
$$N{O_2}$$ is diamagnetic.
240. Which one of the following arrangements does not truly represent the property indicated against it?
A
$$B{r_2} < C{l_2} < {F_2}$$ Oxidising power
B
$$B{r_2} < C{l_2} < {F_2}$$ Electronegativity
C
$$B{r_2} < {F_2} < C{l_2}$$ Electron affinity
D
$$B{r_2} < C{l_2} < {F_2}$$ Bond energy
Answer :
$$B{r_2} < C{l_2} < {F_2}$$ Bond energy