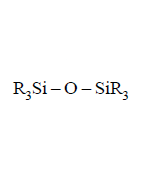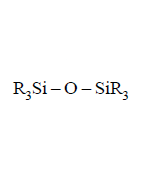211. Which of the following metals does not show inert pair effect?
A
$$Tl$$
B
$$Ga$$
C
$$In$$
D
$$Al$$
Answer :
$$Al$$
212.
$$N{H_4}Cl{O_4} + HN{O_3}\left( {dil.} \right) \to $$ $$HCl{O_4} + \left[ X \right]$$
\[\left[ X \right]\xrightarrow{\Delta }Y\left( g \right)\]
$$\left[ X \right]$$ and $$\left[ Y \right]$$ are respectively -
A
$$N{H_4}N{O_3}\,\,\& \,\,{N_2}O$$
B
$$N{H_4}N{O_2}\,\,\& \,\,{N_2}$$
C
$$HN{O_4}\,\,\& \,\,{O_2}$$
D
$${\text{None of these}}$$
Answer :
$$N{H_4}N{O_3}\,\,\& \,\,{N_2}O$$
213. Mark the correct statements about halogens.
A
Electron affinity of halogens is in the order $$F > Cl > Br > I.$$
B
$$HF$$ is the strongest hydrohalic acid.
C
$${F_2}$$ has lower bond dissociation energy than $$C{l_2}.$$
D
All halogens show variable oxidation states.
Answer :
$${F_2}$$ has lower bond dissociation energy than $$C{l_2}.$$
214. An example of a double salt is
A
bleaching powder
B
$${K_4}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
C
hypo
D
potash alum
Answer :
potash alum
215. Which of the following is a cyclic phosphate ?
A
$${H_3}{P_3}{O_{10}}$$
B
$${H_6}{P_4}{O_{13}}$$
C
$${H_5}{P_5}{O_{15}}$$
D
$${H_7}{P_5}{O_{16}}$$
Answer :
$${H_5}{P_5}{O_{15}}$$
216. Boric acid is a weak monobasic acid and acts as Lewis acid
A
By donating $${H^ + }$$
B
By accepting $$O{H^ - }$$
C
By donating lone pair of electrons
D
By accepting lone pair of electrons.
Answer :
By accepting $$O{H^ - }$$
217. In the structure of diborane
A
all hydrogen atoms lie in one plane and boron atoms lie in a plane perpendicular to this plane
B
2 boron atoms and 4 terminal hydrogen atoms lie in the same plane and 2 bridging hydrogen atoms lie in the perpendicular plane
C
4 bridging hydrogen atoms and boron atoms lie in one plane and two terminal hydrogen atoms lie in a plane perpendicular to this plane
D
all the atoms are in the same plane
Answer :
2 boron atoms and 4 terminal hydrogen atoms lie in the same plane and 2 bridging hydrogen atoms lie in the perpendicular plane
218. Ammonia is a Lewis base. It forms complexes with cations. Which one of the following cations does not form complex with ammonia?
A
$$A{g^ + }$$
B
$$C{u^{2 + }}$$
C
$$C{d^{2 + }}$$
D
$$P{b^{2 + }}$$
Answer :
$$P{b^{2 + }}$$
219. $$Ge\left( {{\text{II}}} \right)$$ compounds are powerful reducing agents whereas $$Pb\left( {{\text{IV}}} \right)$$ compounds are strong oxidants. It is because
A
$$Pb$$ is more electropositive than $$Ge$$
B
ionization potential of lead is less than that of $$Ge$$
C
ionic radii of $$P{b^{2 + }}$$ and $$P{b^{4 + }}$$ are larger than
those of $$G{e^{2 + }}$$ and $$G{e^{4 + }}$$
D
of more pronounced inert pair effect in lead than in $$Ge$$
Answer :
of more pronounced inert pair effect in lead than in $$Ge$$
220. On controlled hydrolysis and condensation, $${R_3}SiCl$$ yields
A
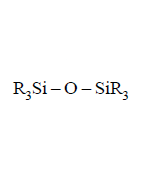
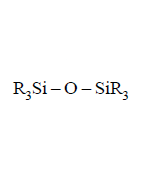
B
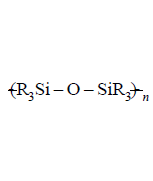
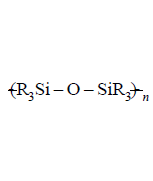
C
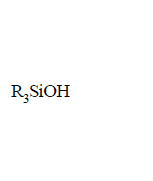
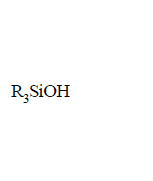
D
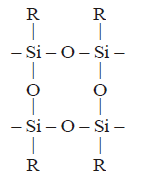
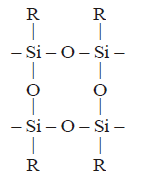
Answer :