201. The structure and hybridisation of $$Si{\left( {C{H_3}} \right)_4}$$ is
A
octahedral, $$s{p^3}d$$
B
tetrahedral, $$s{p^3}$$
C
bent, $$sp$$
D
trigonal, $$s{p^2}$$
Answer :
tetrahedral, $$s{p^3}$$
202. $$B{F_3}$$ is used as a catalyst in various organic reactions because
A
it is a strong reducing agent
B
it is a highly reactive compound
C
it is a weak Lewis acid
D
it is a strong Lewis acid
Answer :
it is a strong Lewis acid
203. Each of the following is true for white and red phosphorus except that they
A
are both soluble in $$C{S_2}$$
B
can be oxidized by heating in air
C
consist of the same kind of atoms
D
can be converted into one another
Answer :
are both soluble in $$C{S_2}$$
204. Which of the following properties of $${{H_2}S{O_4}}$$ in respective reaction is wrong?
A
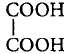 \[\xrightarrow{{{H}_{2}}S{{O}_{4}}}CO+C{{O}_{2}}+{{H}_{2}}O;\] \[{{H}_{2}}S{{O}_{4}}\] acts as dehydrating agent.
\[\xrightarrow{{{H}_{2}}S{{O}_{4}}}CO+C{{O}_{2}}+{{H}_{2}}O;\] \[{{H}_{2}}S{{O}_{4}}\] acts as dehydrating agent.
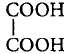 \[\xrightarrow{{{H}_{2}}S{{O}_{4}}}CO+C{{O}_{2}}+{{H}_{2}}O;\] \[{{H}_{2}}S{{O}_{4}}\] acts as dehydrating agent.
\[\xrightarrow{{{H}_{2}}S{{O}_{4}}}CO+C{{O}_{2}}+{{H}_{2}}O;\] \[{{H}_{2}}S{{O}_{4}}\] acts as dehydrating agent. B
$$Cu + 2{H_2}S{O_4} \to CuS{O_4} + 2{H_2}O + S{O_2};$$ $${{H_2}S{O_4}}$$ acts as oxidising agent.
C
$$N{a_2}S + {H_2}S{O_4} \to N{a_2}S{O_4} + {H_2}S;$$ $${{H_2}S{O_4}}$$ acts as an acid.
D
$$2HBr + {H_2}S{O_4} \to 2{H_2}O + S{O_2} + B{r_2};$$ $${{H_2}S{O_4}}$$ acts as reducing agent.
Answer :
$$2HBr + {H_2}S{O_4} \to 2{H_2}O + S{O_2} + B{r_2};$$ $${{H_2}S{O_4}}$$ acts as reducing agent.
205. A type of zeolite used to convert alcohols directly into gasoline is
A
$${\text{zeolite }}A$$
B
$${\text{zeolite }}L$$
C
$${\text{zeolite Beta}}$$
D
$$ZSM - 5$$
Answer :
$$ZSM - 5$$
206. Which of the following acids forms three series of salts?
A
$${H_3}P{O_2}$$
B
$${H_3}B{O_3}$$
C
$${H_3}P{O_4}$$
D
$${H_3}P{O_3}$$
Answer :
$${H_3}P{O_4}$$
207. The shape of the molecule $$S{F_3}C{l_3}$$ is
A
trigonal bipyramidal
B
cubic
C
octahedral
D
tetrahedral
Answer :
octahedral
208. $$Sb{F_5}$$ reacts with $$Xe{F_4}$$ to form an adduct. The shapes of cation and anion in the adduct are respectively :
A
square planar, trigonal bipyramidal
B
$$T$$-shaped, octahedral
C
square pyramidal, octahedral
D
square planar, octahedral
Answer :
$$T$$-shaped, octahedral
209. Which one of the following substances has the highest proton affinity ?
A
$${H_2}S$$
B
$$N{H_3}$$
C
$$P{H_3}$$
D
$${H_2}O$$
Answer :
$$N{H_3}$$
210. A solid element ( symbol $$Y$$ ) conducts electricity and forms two chlorides $$YC{l_n}$$ ( colourless volatile liquid ) and $$YC{l_{n - 2}}$$ ( a colourless solid ). To which one of the following groups of the periodic table does $$Y$$ belong ?
A
13
B
14
C
15
D
16
Answer :
14