331.
Few isomers are given below. Mark the correct statement regarding them.
$$\eqalign{
& \left( {\text{i}} \right)\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]C{l_3} \cr
& \left( {{\text{ii}}} \right)\left[ {Cr{{\left( {{H_2}O} \right)}_5}Cl} \right]C{l_2} \cdot {H_2}O \cr
& \left( {{\text{iii}}} \right)\left[ {Cr{{\left( {{H_2}O} \right)}_4}C{l_2}} \right]Cl \cdot 2{H_2}O \cr} $$
A
(i), (ii) and (iii) are hydrate isomers.
B
(i), (ii) and (iii) are coordination isomers.
C
(i), (ii) and (iii) are ionisation isomers.
D
(i) and (ii) are stereoisomers.
Answer :
(i), (ii) and (iii) are hydrate isomers.
332.
Among the following, which are ambidentate ligands.
$$\eqalign{
& \left( {\text{i}} \right)SC{N^ - } \cr
& \left( {{\text{ii}}} \right)NO_3^ - \cr
& \left( {{\text{iii}}} \right)NO_2^ - \cr
& \left( {{\text{iv}}} \right){C_2}O_4^{2 - } \cr} $$
A
(i) and (iii)
B
(i) and (iv)
C
(ii) and (iii)
D
(ii) and (iv)
Answer :
(i) and (iii)
333. Incorrect match is :
A
$$\left[ {Rh{{\left( {PP{h_3}} \right)}_3}Cl} \right]:$$ Wilkinson's catalyst
B
$${\left[ {Co{{\left( {CO} \right)}_4}} \right]^ - }:$$ Bond order of $$Co—CO$$ bond is greater than one
C
$$\left[ {Zn{{\left( {N{H_3}} \right)}_4}} \right]\left[ {Be{{\left( {OH} \right)}_4}} \right]:$$ colorless complex
D
$${\left[ {Cr{{\left( {CN} \right)}_6}} \right]^{3 - }}:$$ Inner orbital low spin complex
Answer :
$${\left[ {Cr{{\left( {CN} \right)}_6}} \right]^{3 - }}:$$ Inner orbital low spin complex
334. Which one of the following complexes will consume more equivalents of aqueous solution of $$AgN{O_3}?$$
A
$$N{a_2}\left[ {CrC{l_5}\left( {{H_2}O} \right)} \right]$$
B
$$N{a_3}\left[ {CrC{l_6}} \right]$$
C
$$\left[ {Cr{{\left( {{H_2}O} \right)}_5}Cl} \right]C{l_2}$$
D
$$\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]C{l_3}$$
Answer :
$$\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]C{l_3}$$
335. In the coordination compound, $${K_4}\left[ {Ni{{\left( {CN} \right)}_4}} \right],$$ the oxidation state of nickel is
A
$$0$$
B
$$ + 1$$
C
$$ + 2$$
D
$$ - 1$$
Answer :
$$0$$
336.
Mark the correct statements regarding the geometry of complex ions.
(i) The geometry of the complex ion depends upon the coordination number.
(ii) If coordination number is 6, the complex is octahedral.
(iii) If coordination number is 4, the geometry of the complex may be tetrahedral or square planar.
A
(i), (ii) and (iii)
B
(i) and (ii) only
C
(i) and (iii) only
D
(ii) and (iii) only
Answer :
(i), (ii) and (iii)
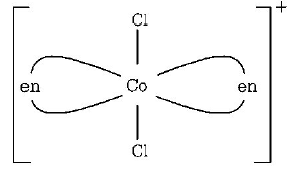
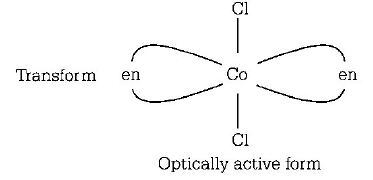
337. Which one of the following is expected to exhibit optical isomerism ? ( $$en =$$ ethylenediamine )
A
$$Cis - \left[ {Pt{{\left( {N{H_3}} \right)}_2}C{l_2}} \right]$$
B
$$Trans - {\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$$
C
$$Trans - \left[ {Pt{{\left( {N{H_3}} \right)}_2}C{l_2}} \right]$$
D
$$Cis - {\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$$
Answer :
$$Cis - {\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$$
338. Which of the following pairs represent linkage isomers?
A
$$\left[ {Pd{{\left( {P\,P{h_3}} \right)}_2}{{\left( {NCS} \right)}_2}} \right]\,{\text{and}}\,\left[ {Pd{{\left( {P\,P{h_3}} \right)}_2}{{\left( {SCN} \right)}_2}} \right]$$
B
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}N{O_3}} \right]S{O_4}\,{\text{and}}\,\left[ {Co{{\left( {N{H_3}} \right)}_5}S{O_4}} \right]N{O_3}$$
C
$$\left[ {PtC{l_2}{{\left( {N{H_3}} \right)}_4}} \right]B{r_2}\,{\text{and}}\,\left[ {Pt\,B{r_2}{{\left( {N{H_3}} \right)}_4}} \right]C{l_2}$$
D
$$\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]\left[ {Pt\,C{l_4}} \right]\,{\text{and}}\,\left[ {Pt{{\left( {N{H_3}} \right)}_4}} \right]\left[ {CuC{l_4}} \right]$$
Answer :
$$\left[ {Pd{{\left( {P\,P{h_3}} \right)}_2}{{\left( {NCS} \right)}_2}} \right]\,{\text{and}}\,\left[ {Pd{{\left( {P\,P{h_3}} \right)}_2}{{\left( {SCN} \right)}_2}} \right]$$
339.
Which of the following type of isomerism is shown by given complex compound ?
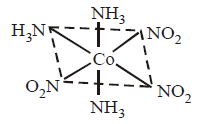
A
Facial
B
Meridional
C
Cis
D
Both (B) and (C)
Answer :
Meridional
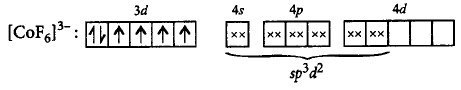
340. $${\left[ {Co{F_6}} \right]^{3 - }}$$ is
A
paramagnetic and undergoes $$s{p^3}{d^2}$$ hybridisation
B
diamagnetic and undergoes $${d^2}s{p^3}$$ hybridisation
C
paramagnetic and undergoes $$s{p^3}d$$ hybridisation
D
diamagnetic and undergoes $$s{p^3}$$ hybridisation
Answer :
paramagnetic and undergoes $$s{p^3}{d^2}$$ hybridisation