321.
Consider the following complex ions, $$P,Q$$ and $$R.$$
$$P = {\left[ {Fe{F_6}} \right]^{3 - }},Q = {\left[ {V{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}{\text{and}}\,R = {\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
The correct order of the complex ions, according to their spin-only magnetic moment values $$\left( {{\text{in}}\,{\text{B}}{\text{.M}}.} \right)$$ is
A
$$R < Q < P$$
B
$$Q < R < P$$
C
$$R < P < Q$$
D
$$Q < P < R$$
Answer :
$$Q < R < P$$
322.
Which of the following has a square planar geometry?
$$\left( {{\text{At}}{\text{.}}\,{\text{nos}}.:Fe = 26,Co = 27,Ni = 28,Pt = 78} \right)$$
A
$${\left[ {PtC{l_4}} \right]^{2 - }}$$
B
$${\left[ {CoC{l_4}} \right]^{2 - }}$$
C
$${\left[ {FeC{l_4}} \right]^{2 - }}$$
D
$${\left[ {NiC{l_4}} \right]^{2 - }}$$
Answer :
$${\left[ {PtC{l_4}} \right]^{2 - }}$$
323. Both geometrical and optical isomerism are shown by
A
$${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$$
B
$${\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]^{2 + }}$$
C
$${\left[ {Co{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]^ + }$$
D
$${\left[ {Cr{{\left( {ox} \right)}_3}} \right]^{3 - }}$$
Answer :
$${\left[ {Co{{\left( {en} \right)}_2}C{l_2}} \right]^ + }$$
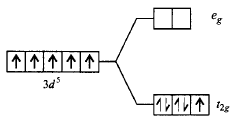
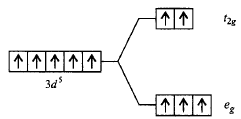
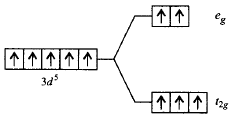
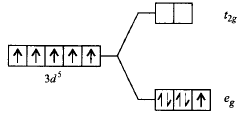
324. Which of the following energy level diagram for $${\left[ {Fe{F_6}} \right]^{3 - }}$$ is correct on the basis of crystal field theory?
A

B

C

D

Answer :


325. The correct IUPAC name of $$\left[ {Pt{{\left( {N{H_3}} \right)}_2}C{l_2}} \right]$$ is
A
diamminedichloridoplatinum(II)
B
diamminedichloridoplatinum(IV)
C
diamminedichloridoplatinum(0)
D
dichloridodiammineplatinum(IV)
Answer :
diamminedichloridoplatinum(II)
326. As per IUPAC nomenclature, the name of the complex $$\left[ {Co{{\left( {{H_2}O} \right)}_4}{{\left( {N{H_3}} \right)}_2}} \right]C{l_3}\,{\text{is}}:$$
A
Tetraaquadiaminecobalt (III) chloride
B
Tetraaquadiamminecobalt (III) chloride
C
Diaminetetraaquacoblat (III) chloride
D
Diamminetetraaquacobalt (III) chloride
Answer :
Diamminetetraaquacobalt (III) chloride
327. The unpaired electrons in $$Ni{\left( {CO} \right)_4}$$ are
A
zero
B
one
C
three
D
four
Answer :
zero
328. A solution containing $$2.675g$$ of a cobalt (III) chloride ammonia complex ( molar mass $$ = 267.5\,g\,mo{l^{ - 1}}$$ ) is passed through a cation exchanger. The chloride ions obtained in solution were treated with excess of $$AgN{O_3}$$ to give $$4.78\,g$$ of $$AgCl$$ ( molar mass = $$143.5\,g\,mo{l^{ - 1}}$$ ). The formula of the complex is (At. mass of $$Ag = 108\,u$$ )
A
$$\left[ {CoCl{{\left( {N{H_3}} \right)}_5}} \right]C{l_2}$$
B
$$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}$$
C
$$\left[ {CoC{l_2}{{\left( {N{H_3}} \right)}_4}} \right]Cl$$
D
$$\left[ {CoC{l_3}{{\left( {N{H_3}} \right)}_3}} \right]$$
Answer :
$$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}$$
329. One mole of the complex compound $$Co{\left( {N{H_3}} \right)_5}C{l_3},$$ gives $$3$$ moles ofions on dissolution in water. One mole of the same complex reacts with two moles of $$AgN{O_3}$$ solution to yield two moles of $$AgCl\left( s \right).$$ The structure of the complex is
A
$$\left[ {Co{{\left( {N{H_3}} \right)}_3}C{l_3}} \right].2\,N{H_3}$$
B
$$\left[ {Co{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]Cl.\,N{H_3}$$
C
$$\left[ {Co{{\left( {N{H_3}} \right)}_4}Cl} \right]C{l_2}.\,N{H_3}$$
D
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]C{l_2}$$
Answer :
$$\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]C{l_2}$$
330. A chelating agent has two or more than two donor atoms to bind to a single metal ion. Which of the following is not a chelating agent?
A
Thiosulphato
B
Oxalato
C
Glycinato
D
Ethane - 1, 2 - diamine
Answer :
Thiosulphato
.PNG)
.PNG)
