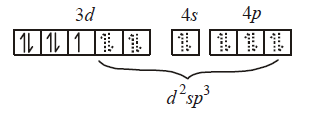
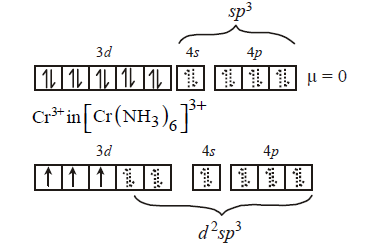
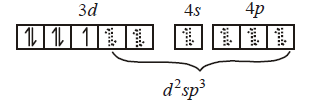
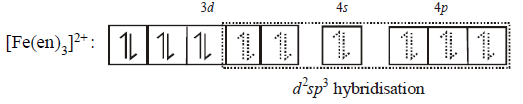
291. Which molecule/ion among the following cannot act as a ligand in complex compounds ?
A
$$C{H_4}$$
B
$$CO$$
C
$$C{N^ - }$$
D
$$B{r^ - }$$
Answer :
$$C{H_4}$$
292. Which of the following shall form an octahedral complex?
A
$${d^4}{\text{(low spin)}}$$
B
$${d^8}{\text{(high spin)}}$$
C
$${d^6}{\text{(low spin)}}$$
D
$${\text{None of these}}$$
Answer :
$${d^6}{\text{(low spin)}}$$
293.
Which of the following complex compound$$(s)$$ is/are paramagnetic and low spin ?
$$\eqalign{
& \left( {\text{i}} \right){K_3}\left[ {Fe\left( {C{N_6}} \right)} \right] \cr
& \left( {{\text{ii}}} \right){\left[ {Ni{{\left( {CO} \right)}_4}} \right]^0} \cr
& \left( {{\text{iii}}} \right){\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }} \cr
& \left( {{\text{iv}}} \right)\left[ {Mn{{\left( {CN} \right)}_6}} \right] \cr} $$
Choose the correct code :
A
(i) only
B
(ii) and (iii)
C
(i) and (iv)
D
(iv) only
Answer :
(i) and (iv)
294. Identify the statement which is not correct.
A
Coordination compounds are mainly known for transition metals.
B
Coordination number and oxidation state of a metal are same.
C
A ligand donates at least one electron pair to the metal atom to form a bond.
D
$${\left[ {Co{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]^ + }$$ is a heteroleptic complex.
Answer :
Coordination number and oxidation state of a metal are same.
295. $${\left[ {Fe{{\left( {en} \right)}_2}{{\left( {{H_2}O} \right)}_2}} \right]^{2 + }} + en \to $$ $${\text{complex }}(X){\text{.}}$$ The correct statement about the complex $$(X)$$ is –
A
it is a low spin complex
B
it is diamagnetic
C
it shows geometrical isomerism
D
(A) and (B) both
Answer :
(A) and (B) both
296.
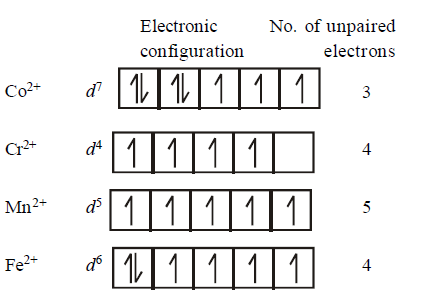
The $$d$$ - electron configurations of $$C{r^{2 + }},M{n^{2 + }},F{e^{2 + }}$$ and $$C{o^{2 + }}$$ are $${d^4},{d^5},{d^6}$$ and $${d^7}$$ respectively. Which one of the following will exhibit the lowest paramagnetic behaviour ?
$$\left( {{\text{Atomic no}}{\text{. }}Cr = {\text{ }}24,Mn = 25,Fe = 26,Co = 27} \right).$$
A
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
B
$${\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
C
$${\left[ {Mn{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
D
$${\left[ {Fe{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
Answer :
$${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$
297. Which of the following species is not expected to be a ligand?
A
$$NO$$
B
$$NH_4^ + $$
C
$$N{H_2}C{H_2}C{H_2}N{H_2}$$
D
$$CO$$
Answer :
$$NH_4^ + $$
298. When $$0.1\,mol\,CoC{l_3}{\left( {N{H_3}} \right)_5}$$ is treated with excess of $$AgN{O_3},0.2\,mol$$ of $$AgCl$$ are obtained. The conductivity of solution will correspond to
A
1 : 3 electrolyte
B
1 : 2 electrolyte
C
1 : 1 electrolyte
D
3 : 1 electrolyte
Answer :
1 : 2 electrolyte
299. Among the following, the coloured compound is
A
$$CuCl$$
B
$${K_3}\left[ {Cu{{\left( {CN} \right)}_4}} \right]$$
C
$$Cu{F_2}$$
D
$$\left[ {Cu{{\left( {C{H_3}CN} \right)}_4}} \right]B{F_4}$$
Answer :
$$Cu{F_2}$$
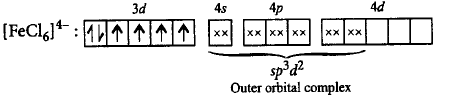
300. Which of the following descriptions about $${\left[ {FeC{l_6}} \right]^{4 - }}$$ is correct about the complex ion?
A
$$s{p^3}d,$$ inner orbital complex, diamagnetic
B
$$s{p^3}{d^2},$$ outer orbital complex, paramagnetic
C
$${d^2}s{p^3},$$ inner orbital complex, paramagnetic
D
$${d^2}s{p^3},$$ outer orbital complex, diamagnetic
Answer :
$$s{p^3}{d^2},$$ outer orbital complex, paramagnetic