211. The complex $$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Cr{{\left( {CN} \right)}_6}} \right]$$ and $$\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Co{{\left( {CN} \right)}_6}} \right]$$ are the examples of which type of isomerism ?
A
lonisation isomerism
B
Coordination isomerism
C
Geometrical isomerism
D
Linkage isomerism
Answer :
Coordination isomerism
212. Which is not a $$\pi $$ - acceptor ligand ?
A
$$I_3^ - $$
B
$$N{O^ + }$$
C
$${\left( {C{H_3}} \right)_3}P$$
D
$$C{N^ - }$$
Answer :
$$N{O^ + }$$
213. The value of the ‘spin only’ magnetic moment for one of the following configurations is 2.84 $$BM.$$ The correct one is
A
$${d^5}$$ (in strong ligand field)
B
$${d^3}$$ (in weak as well as in strong fields)
C
$${d^4}$$ (in weak ligand fields)
D
$${d^4}$$ (in strong ligand fields)
Answer :
$${d^4}$$ (in strong ligand fields)
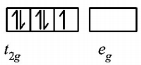
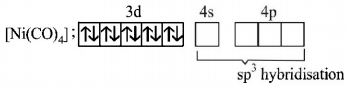
214. Both $$\left[ {Ni{{\left( {CO} \right)}_4}} \right]\,\,{\text{and}}\,\,{\left[ {Ni{{\left( {CN} \right)}_4}} \right]^{2 - }}$$ are diamagnetic. The hybridisation of nickel in these complexes,respectively,
A
$$s{p^3},s{p^3}$$
B
$$s{p^3},ds{p^2}$$
C
$$ds{p^2},s{p^3}$$
D
$$ds{p^2},s{p^2}$$
Answer :
$$s{p^3},ds{p^2}$$
215. The magnitude of $$CFSE$$ ( crystal field splitting energy, $${\Delta _o}$$ ) can be related to the configuration of $$d$$ - orbitals in a coordination entity as
A
If $${\Delta _o} < P,$$ the configuration is $$t_{2g}^3\,e_g^1 = $$ weak field ligand and high spin complex
B
if $${\Delta _o} > P,$$ the configuration is $$t_{2g}^3\,e_g^1 = $$ strong field ligand and low spin complex
C
if $${\Delta _o} > P,$$ the configuration is $$t_{2g}^4\,e_g^0 = $$ strong field ligand and high spin complex
D
if $${\Delta _o} = P,$$ the configuration is $$t_{2g}^4\,e_g^0 = $$ strong field ligand and high spin complex.
Answer :
If $${\Delta _o} < P,$$ the configuration is $$t_{2g}^3\,e_g^1 = $$ weak field ligand and high spin complex
216. The magnitude of magnetic moment (spin only) of $${\left[ {NiC{l_4}} \right]^{2 - }}$$ will be
A
2.82 $$B.M.$$
B
3.25 $$B.M.$$
C
1.23 $$B.M.$$
D
5.64 $$B.M.$$
Answer :
2.82 $$B.M.$$
217. The compounds $$\left[ {Co\left( {S{O_4}} \right){{\left( {N{H_3}} \right)}_5}} \right]Br$$ and $$\left[ {Co\left( {S{O_4}} \right){{\left( {N{H_3}} \right)}_5}} \right]Cl$$ represent
A
linkage isomerism
B
ionisation isomerism
C
coordination isomerism
D
no isomerism
Answer :
no isomerism
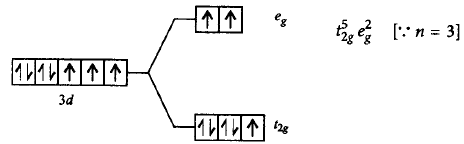
218. Which of the following statements is correct about $${\left[ {Co{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}$$ complex?
A
Electronic configuration $$ = 3{d^7} \to t_{2g}^5\,e_g^2,$$ no. of unpaired electrons $$ = 3,\mu = 3.87\,B.M.$$
B
Electronic configuration $$ = 3{d^6} \to t_{2g}^4\,e_g^2,$$ no. of unpaired electrons $$ = 2,\mu = 2.87\,B.M.$$
C
Electronic configuration $$ = 3{d^7} \to t_{2g}^6\,e_g^1,$$ no. of unpaired electrons $$ = 1,\mu = 2.87\,B.M.$$
D
Electronic configuration $$ = 3{d^7} \to t_{2g}^3\,e_g^4,$$ no. of unpaired electrons $$ = 3,\mu = 3.87\,B.M.$$
Answer :
Electronic configuration $$ = 3{d^7} \to t_{2g}^5\,e_g^2,$$ no. of unpaired electrons $$ = 3,\mu = 3.87\,B.M.$$
219.
The degeneracy of $$d$$ - orbitals is lost under :
(i) Strong field ligand
(ii) Weak field ligand
(iii) Mixed field ligand
(iv) Chelated ligand field
A
(i), (ii) and (iv)
B
(i) and (ii)
C
(i), (ii), (iii) and (iv)
D
(i), (ii) and (iii)
Answer :
(i), (ii), (iii) and (iv)
220. Select the complex in which secondary valency is satisfied before the primary valency.
A
$$RMgX$$
B
$$\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]C{l_2}$$
C
$${K_4}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
D
$$\left[ {Ni{{\left( {CO} \right)}_4}} \right]$$
Answer :
$$\left[ {Ni{{\left( {CO} \right)}_4}} \right]$$
.PNG)
.PNG)
.PNG)
.PNG)