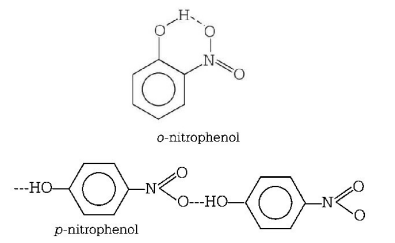
371. The boiling point of $$p$$ - nitrophenol is higher than that of $$o$$ - nitrophenol because
A
$$N{O_2}$$ group at $$p$$ - position behave in a different way from that at $$o$$ - position
B
intramolecular hydrogen bending exists in $$p$$ - nitrophenol
C
there is intermolecular hydrogen bonding in $$p$$ - nitrophenol
D
$$p$$ - nitrophenol has a higher molecular weight than $$o$$ - nitrophenol
Answer :
there is intermolecular hydrogen bonding in $$p$$ - nitrophenol
372. Sodium chloride has a crystalline structure made up of $$N{a^ + }$$ and $$C{l^ - }$$ ions. Why does $$NaCl$$ not conduct electricity in solid state?
A
The ions of $$NaCl$$ become mobile only in molten state and are not free to move in solid state.
B
The crystalline structure does not have ions.
C
When a bond is formed between ions they lose their charges.
D
None of these.
Answer :
The ions of $$NaCl$$ become mobile only in molten state and are not free to move in solid state.
373. Which one of the following molecules is paramagnetic?
A
$${N_2}$$
B
$$NO$$
C
$$CO$$
D
$${O_3}$$
Answer :
$$NO$$
374.
The true statements from the following are
1. $$P{H_5}$$ and $$BiC{l_5}$$ do not exist
2. $$p\pi - d\pi $$ bond is present in $$S{O_2}$$
3. Electrons travel with the speed of light
4. $$Se{F_4}$$ and $$C{H_4}$$ have same shape
5. $$I_3^ + $$ has bent geometry
A
1, 3
B
1, 2, 5
C
1, 3, 5
D
1, 2, 4
Answer :
1, 2, 5
375. Which of the following is the wrong statement?
A
$$ONCl$$ and $$ON{O^ - }$$ are not isoelectronic.
B
$${O_3}$$ molecule is bent
C
Ozone is violet-black in solid state
D
Ozone is diamagnetic gas.
Answer :
$$ONCl$$ and $$ON{O^ - }$$ are not isoelectronic.
376. The electronic configuration of the outermost shell of the most electronegative element is
A
$$2{s^2}2{p^5}$$
B
$$3{s^2}3{p^5}$$
C
$$4{s^2}4{p^5}$$
D
$$5{s^2}5{p^5}$$
Answer :
$$2{s^2}2{p^5}$$
377. Geometrical shapes of the complexes formed by the reaction of $$N{i^{2 + }}$$ with $$C{l^ - },\,C{N^ - }\,$$ and $${H_2}O,$$ respectively,are
A
octahedral, tetrahedral and square planar
B
tetrahedral, square planar and octahedral
C
square planar, tetrahedral and octahedral
D
octahedral, square planar and octahedral
Answer :
tetrahedral, square planar and octahedral
378. The hybridization of orbitals of $$N$$ atom in $$NO_3^ - ,\,NO_2^ + $$ and $$NH_4^ + $$ are respectively:
A
$$sp,s{p^2}s{p^3}$$
B
$$s{p^2},sp,s{p^3}$$
C
$$sp,s{p^3},s{p^2}$$
D
$$s{p^2},s{p^3},sp$$
Answer :
$$s{p^2},sp,s{p^3}$$
379. Which of the following pairs have identical bond order ?
A
$${N_2},O_2^{2 + }$$
B
$${N_2},O_2^ - $$
C
$$N_2^ - ,{O_2}$$
D
$${O^{2 + }},{N_2}$$
Answer :
$${N_2},O_2^{2 + }$$
380. Which of the following is not a correct statement?
A
Ionic compounds are electrically neutral.
B
Boiling point of an ionic compound is more than a covalent compound.
C
Melting point of a covalent compound is more than an ionic compound.
D
Ionic compounds are soluble in polar solvent.
Answer :
Melting point of a covalent compound is more than an ionic compound.
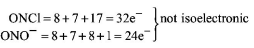

.PNG)
.PNG)
.PNG)
.PNG)
.PNG)
.PNG)
.PNG)