361. Among the following compounds the one that is polar and has the central atom with $$s{p^2}$$ hybridisation is
A
$${H_2}C{O_3}$$
B
$$Si{F_4}$$
C
$$B{F_3}$$
D
$$HCl{O_2}$$
Answer :
$${H_2}C{O_3}$$
362. Among the following orbital bonds, the angle is minimum between
A
$$s{p^3}\,{\text{bonds}}$$
B
$${p_x}\,\,{\text{and}}\,{p_y} - {\text{orbitals}}$$
C
$$H - O - H\,{\text{in}}\,{\text{water}}$$
D
$$sp\,{\text{bonds}}$$
Answer :
$${p_x}\,\,{\text{and}}\,{p_y} - {\text{orbitals}}$$
363. Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of the following has the highest dipole moment?
A
$$C{O_2}$$
B
$$HI$$
C
$${H_2}O$$
D
$$S{O_2}$$
Answer :
$${H_2}O$$
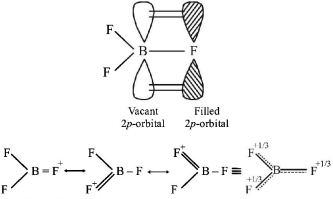
364. The bond dissociation energy of $$B - F\,\,{\text{in}}\,\,{\text{B}}{{\text{F}}_3}$$ is $$646\,\,kJ\,\,mo{l^{ - 1}}$$ whereas that of $$C - F\,\,{\text{in}}\,\,C{F_4}\,\,{\text{is}}\,\,515\,kJ\,\,mo{l^{ - 1}}.\,\,$$ The correct reason for higher $$B - F$$ bond dissociation energy as compared to that of $$C - F$$ is
A
stronger $$\sigma $$ bond between $$B\,{\text{and}}\,F\,\,{\text{in}}\,\,B{F_3}$$ as compared to that between $$C\,{\text{and}}\,F\,\,{\text{in}}\,\,C{F_4}.$$
B
significant $$p\pi - p\pi $$ interaction between $$B\,{\text{and}}\,F\,\,{\text{in}}\,\,B{F_3}$$ whereas there is no possibility of such interaction between $$C\,{\text{and}}\,F\,\,{\text{in}}\,\,C{F_4}.$$
C
lower degree of $$p\pi - p\pi $$ interaction between $$B\,{\text{and}}\,F\,\,{\text{in}}\,\,B{F_3}$$ than that between $$C\,{\text{and}}\,F\,\,{\text{in}}\,\,C{F_4}.$$
D
smaller size of $$B - {\text{atom}}$$ as compared to that of $$C - {\text{atom}}$$
Answer :
significant $$p\pi - p\pi $$ interaction between $$B\,{\text{and}}\,F\,\,{\text{in}}\,\,B{F_3}$$ whereas there is no possibility of such interaction between $$C\,{\text{and}}\,F\,\,{\text{in}}\,\,C{F_4}.$$
365.
The given structures I, II and III of carbonate ion represent :
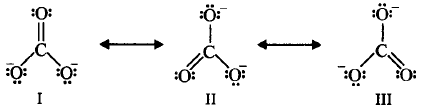
A
hybrid structures
B
isomeric structures
C
canonical structures
D
dipole structures
Answer :
canonical structures
366. Which of the correct increasing order of lone pair of electrons on the central atom?
A
$$I{F_7} < I{F_5} < Cl{F_3} < Xe{F_2}$$
B
$$I{F_7} < Xe{F_2} < Cl{F_2} < I{F_5}$$
C
$$I{F_7} < Cl{F_3} < Xe{F_2} < I{F_5}$$
D
$$I{F_7} < Xe{F_2} < I{F_5} < Cl{F_3}$$
Answer :
$$I{F_7} < I{F_5} < Cl{F_3} < Xe{F_2}$$
367. Which of the following molecules contains covalent and coordinate bonds?
A
$$CC{l_4}$$
B
$${H_2}S{O_4}$$
C
$$NaCl$$
D
$$Mg{\left( {OH} \right)_2}$$
Answer :
$${H_2}S{O_4}$$
368. Which types of bonds are present between two carbon atoms in acetylene molecule?
A
Two $$sigma$$ bonds and one $$pi$$ bond
B
Three $$sigma$$ bonds
C
One $$sigma$$ bond and two $$pi$$ bonds
D
Three $$pi$$ bonds
Answer :
One $$sigma$$ bond and two $$pi$$ bonds
369. Which of the following is non-polar?
A
$$S{O_2}$$
B
$$C{O_2}$$
C
$${H_2}O$$
D
$$N{H_3}$$
Answer :
$$C{O_2}$$
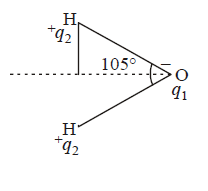
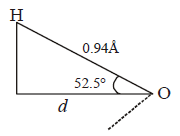
370. Dipole moment of $${H_2}O$$ is $$1.85\,D.$$ If the bond angle is $${105^ \circ }$$ and $$O - H$$ bond length is $$0.94\mathop {\text{A}}\limits^{\text{o}} ,$$ what is the magnitude of charge on the oxygen atom in water molecule?
A
$$2 \times {10^{ - 10}}esu$$
B
$$4.28 \times {10^{ - 10}}esu$$
C
$$3.22 \times {10^{ - 10}}esu$$
D
$$1.602 \times {10^{ - 19}}C$$
Answer :
$$3.22 \times {10^{ - 10}}esu$$
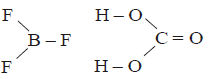
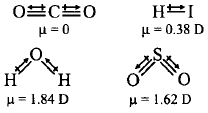
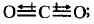 Since the dipoles are equal and acting in opposite directions. Therefore, the net dipole moment is zero.
Since the dipoles are equal and acting in opposite directions. Therefore, the net dipole moment is zero.