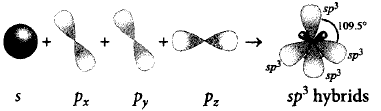331. Which one of the following formulae does not correctly represent the bonding capacities of the atoms involved?
A
.PNG)
.PNG)
B
.PNG)
.PNG)
C
.PNG)
.PNG)
D
.PNG)
.PNG)
Answer :
.PNG)
.PNG)
332. Oxygen molecule is formed by
A
one axial $$s{\text{ - }}s$$ overlap and one $$p{\text{ - }}p$$ axial overlap
B
two $$p{\text{ - }}p$$ axial overlaps
C
two $$p{\text{ - }}p$$ sidewise overlaps
D
one $$p{\text{ - }}p$$ axial and one $$p{\text{ - }}p$$ sidewise overlap
Answer :
one $$p{\text{ - }}p$$ axial and one $$p{\text{ - }}p$$ sidewise overlap
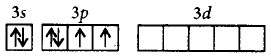
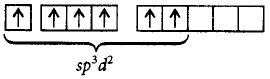
333. The ground state electronic configuration of $$S$$ is $$3{s^2}3{p^4}.$$ How does it form the compound $$S{F_6}?$$
A
Due to octahedral shape of $$S$$ atoms.
B
Due to presence of vacant 3 $$d$$ - orbitals which provide 6 unpaired electrons in excited state.
C
Due to $$s{p^3}$$ hybridisation of $$S$$ atom which provides 6 electrons to 6 $$F$$ atoms.
D
Due to presence of 3 $$sigma$$ and 3 $$pi$$ bonds between $$S$$ and $$F.$$
Answer :
Due to presence of vacant 3 $$d$$ - orbitals which provide 6 unpaired electrons in excited state.
334. Which one of the following compounds has the smallest bond angle in its molecule ?
A
$$O{H_2}\,$$
B
$$S{H_2}$$
C
$$N{H_3}\,$$
D
$$S{O_2}$$
Answer :
$$S{H_2}$$
335. Which of the following options represents the correct bond order?
A
$$O_2^ - > {O_2} > O_2^ + $$
B
$$O_2^ - < {O_2} < O_2^ + $$
C
$$O_2^ - > {O_2} < O_2^ + $$
D
$$O_2^ - < {O_2} > O_2^ + $$
Answer :
$$O_2^ - < {O_2} < O_2^ + $$
336. The electronic configuration of carbon is $$1{s^2}2{s^2}2{p^2}.$$ There are 12 electrons in $${C_2}.$$ The correct electronic configuration of $${C_2}$$ molecule is
A
$$\left( {\sigma 1{s^2}} \right)\left( {{\sigma ^ * }1{s^2}} \right)\left( {\sigma 2{s^2}} \right)\left( {{\sigma ^ * }2{s^2}} \right)$$ $$\left( {\sigma 2p_z^2} \right)\left( {\pi 2p_x^2} \right)$$
B
$$\left( {\sigma 1{s^2}} \right)\left( {{\sigma ^ * }1{s^2}} \right)\left( {\sigma 2{s^2}} \right)\left( {{\sigma ^ * }2{s^2}} \right)$$ $$\left( {\pi 2p_x^2 = \pi 2p_y^2} \right)$$
C
$$\left( {\sigma 1{s^2}} \right)\left( {{\sigma ^ * }1{s^2}} \right)\left( {\sigma 2{s^2}} \right)\left( {{\sigma ^ * }2{s^2}} \right)$$ $$\left( {\sigma 2p_z^2} \right)\left( {\pi 2p_x^1 = \pi 2p_y^1} \right)$$
D
$$\left( {\sigma 1{s^2}} \right)\left( {{\sigma ^ * }1{s^2}} \right)\left( {\sigma 2{s^2}} \right)\left( {{\sigma ^ * }2{s^2}} \right)$$ $$\left( {\pi 2p_x^2 = \pi 2p_y^1} \right)$$
Answer :
$$\left( {\sigma 1{s^2}} \right)\left( {{\sigma ^ * }1{s^2}} \right)\left( {\sigma 2{s^2}} \right)\left( {{\sigma ^ * }2{s^2}} \right)$$ $$\left( {\pi 2p_x^2 = \pi 2p_y^2} \right)$$
337. Which of the following does not have a tetrahedral structure?
A
$$BH_4^ - $$
B
$$B{H_3}$$
C
$$NH_4^ + $$
D
$${H_2}O$$
Answer :
$$B{H_3}$$
338. The molecule that has linear structure is
A
$$C{O_2}$$
B
$$N{O_2}$$
C
$$S{O_2}$$
D
$$Si{O_2}$$
Answer :
$$C{O_2}$$
339. On hybridisation of one $$s$$ and three $$p$$ - orbitals, we get
A
four orbitals with tetrahedral orientation
B
three orbitals with trigonal orientation
C
two orbitals with linear orientation
D
two orbitals with perpendicular orientation
Answer :
four orbitals with tetrahedral orientation
340. Which one of the following compounds has $$s{p^2}$$ hydridization?
A
$$C{O_2}$$
B
$$S{O_2}$$
C
$${N_2}O$$
D
$$CO$$
Answer :
$$S{O_2}$$
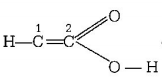 carbon number $$2$$ have five valency which is not possible, so it does not correctly represent the bonding capacities of $$C$$ atom.
carbon number $$2$$ have five valency which is not possible, so it does not correctly represent the bonding capacities of $$C$$ atom.