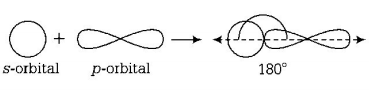
311. The angle between the overlapping of one $$s$$ -orbital and one $$p$$ -orbital is
A
$${180^ \circ }$$
B
$${120^ \circ }$$
C
$${109^ \circ }28'$$
D
$${120^ \circ }60'$$
Answer :
$${180^ \circ }$$
312. Which of the following statements is not correct from the view point of molecular orbital theory?
A
$$B{e_2}$$ is not a stable molecul.
B
$$H{e_2}$$ is not stable but $$He_2^ + $$ is expected to exist.
C
Bond strength of $${N_2}$$ is maximum amongst the homonuclear diatomic molecules belonging to the second period.
D
The order of energies of molecular orbitals in $${N_2}$$ molecule is $$\sigma 2s < {\sigma ^ * }2s < \sigma 2{p_z} < $$ $$\left( {\pi 2{p_x} = \pi 2{p_y}} \right) < \left( {{\pi ^ * }2{p_x} = {\pi ^ * }2{p_y}} \right)$$ $$ < {\sigma ^ * }2{p_z}$$
Answer :
The order of energies of molecular orbitals in $${N_2}$$ molecule is $$\sigma 2s < {\sigma ^ * }2s < \sigma 2{p_z} < $$ $$\left( {\pi 2{p_x} = \pi 2{p_y}} \right) < \left( {{\pi ^ * }2{p_x} = {\pi ^ * }2{p_y}} \right)$$ $$ < {\sigma ^ * }2{p_z}$$
313. Which one is most ionic :
A
$${P_2}{O_5}$$
B
$$\,Cr{O_3}$$
C
$$MnO$$
D
$$M{n_2}{O_7}$$
Answer :
$$MnO$$
314. The geometry and the type of hybrid orbital present about the central atom in $$B{F_3}$$ is
A
linear, $$sp$$
B
trigonal planar, $$s{p^2}$$
C
tetrahedral, $$s{p^3}$$
D
pyramidal, $$s{p^3}$$
Answer :
trigonal planar, $$s{p^2}$$
315. Molecular shapes of $$S{F_4},C{F_4}$$ and $$Xe{F_4}$$ are
A
the same, with 2, 0 and 1 lone pairs of electrons respectively
B
the same, with 1, 1 and 1 lone pairs of electrons respectively
C
different, with 0, 1 and 2 lone pairs of electrons respectively
D
different, with 1, 0 and 2 lone pairs of electrons respectively
Answer :
different, with 1, 0 and 2 lone pairs of electrons respectively
316. The shape of $$IF_6^ - $$ is :
A
Trigonally distorted octahedron
B
Pyramidal
C
Octahedral
D
Square antiprism
Answer :
Trigonally distorted octahedron
317. How many orbitals are singly occupied in $${O_2}$$ molecule?
A
2
B
1
C
3
D
4
Answer :
2
318. An ether is more volatile than an alcohol having the same molecular formula. This is due to
A
alcohols having resonance structures
B
inter-molecular hydrogen bonding in ethers
C
imter-molecular hydrogen bonding in alcohols
D
dipolar character of ethers
Answer :
imter-molecular hydrogen bonding in alcohols
319. Which one of the following has the highest dipole moment?
A
$$As{H_3}$$
B
$$Sb{H_3}$$
C
$$P{H_3}$$
D
$$N{H_3}$$
Answer :
$$N{H_3}$$
320.
Match the bond enthalpies given in column II with the molecules given in column I and mark the appropriate choice.
Column I
Column II
a.
Hydrogen $$\left( {{H_2}} \right)$$
1.
$$498.0\,kJ\,mo{l^{ - 1}}$$
b.
Oxygen $$\left( {{O_2}} \right)$$
2.
$$946.0\,kJ\,mo{l^{ - 1}}$$
c.
Nitrogen $$\left( {{N_2}} \right)$$
3.
$$435.8\,kJ\,mo{l^{ - 1}}$$
A
a - 1, b - 2, c - 3
B
a - 3, b - 2, c - 1
C
a - 1, b - 3, c - 2
D
a - 3, b - 1, c - 2
Answer :
a - 3, b - 1, c - 2